
Sometimes seemingly insignificant details have an incredible impact on the overall picture. This principle is applicable to many areas of our life: the mysterious smile of Mona Lisa, which gave rise to a lot of theories and speculations; one line of code that can completely change the functionality of the program; the order of arrangement of atoms, which changes the properties of a substance. We will talk about the latter today. Scientists from the University of Tsukuba (Japan) put forward a theory according to which it is possible to create a new structure of diamond, which will be harder than the known mineral. Scientists named their creation "penta diamond". What is needed to create a penta diamond, what properties can it possess, and where can such an unusual substance be used? We will find answers to these questions in the report of scientists. Go.
Basis of research
Carbon is an ubiquitous element in the periodic table and is the basis for a great variety of organic and inorganic compounds. In nature, carbon can be found almost everywhere: oil and peat, methane and carbon dioxide, muscles and bones, etc. etc. In short, carbon is considered one of the main building blocks of life on Earth for a reason.
Certain substances or chemical elements can exist in different forms under different conditions. This is called allotropy. Carbon is the record holder in this business, as it has more than 9 allotropic modifications.
The reasons for the presence of such a large number of carbon allotropes are orbital hybridization, boundary conditions, and topological defects. Three forms of orbital hybridization (sp, sp 2 and sp 3) represent allotropes covering all dimensions:
- one-dimensional chains for sp (polyyne);
- two-dimensional sheet for sp 2 (graphene);
- 3D mesh for sp 3 (diamond).
In addition, boundary conditions and topological defects allow carbon to form additional allotropes with unique morphologies: for example, fullerene and carbon nanotubes, which have unusual electronic and structural properties.
A variety of fullerenes.
Carbon allotropes, consisting of sp 2 and sp 3 C atoms, attract the attention of researchers because of their morphological diversity, which is due to the huge number of combinations of sp 2 and sp 3 atoms within one system. An example of this is fullerenes treated with high pressure and temperature (for example, C 60), which can have a low mass density due to covalent bonds between fullerenes and nanosized pores.
In addition, there are studies that describe the rapid quenching of amorphous carbon, which leads to the formation of a hybrid allotrope sp 2 -sp 3 -Q -carbon, which has magnetism and hardness superior to diamond.
Carbon can be roughly called plasticine in the hands of scientists, because from it you can create a lot of substances with different properties and functionality, there would be desire and fantasy.
In this work, scientists presented a theoretical study of a three-dimensional carbon allotrope, consisting of atoms C sp 2 and sp 3(penta diamond). It can be obtained by copolymerization of hydrocarbon molecules containing pentagonal rings (spiro [2.4] heptane-4.6-diene (C 7 H 8 ) and [5.5.5.5] -phenestran-tetraine).
Penta diamond consists of pentagonal rings with space group Fm¯3m due to copolymerization of these constituent molecules. Calculations have shown that penta diamond has a high volume modulus * - 381 GPa, which is approximately 80% of diamond. This suggests that penta diamond is a solid carbon allotrope.
In addition, it has a negative Poisson's ratio * (-0.241), which leads to extremely high Young's modulus * (1691 GPa) and shear modulus * (1113 GPa), which is higher than that of diamond or other ultra-strong carbons.
Bulk modulus of elasticity * - a characteristic of the ability of a substance to resist all-round compression.
Poisson's ratio * - the ratio of the relative lateral compression to the relative longitudinal tension.
Young's modulus * (modulus of elasticity) - an assessment of the ability of a material to resist tension, compression under elastic deformation.
Shear modulus * - an assessment of the material's ability to resist shear deformation.Penta diamond is a semiconductor with an indirect band gap * of 2.25 eV, which is expected to have a high hole mobility * .
* — , .
* — .
All calculations were carried out on the basis of the density functional theory implemented in the STATE software package ( STF-ElectronMo ).
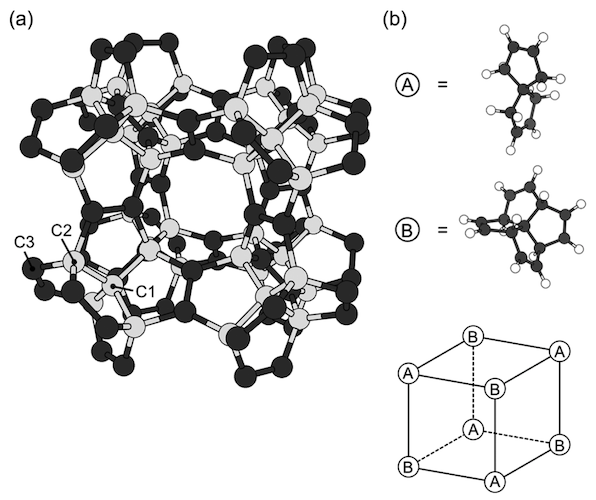
Image # 1
The diagrams above show the optimized geometry of a pentagon with a lattice parameter of 9.195 Å and space group Fm¯3m. The covalent network consists exclusively of pentagons, in which three of the five edges are separated by neighboring pentagons ( 1a ), due to the copolymerization of C 7 H 8 and [5.5.5.5] -phenestran-tetrain, which are alternately located at the vertices of the cubic lattice ( 1b ).
According to these constituent molecules and their arrangement, the unit cell of a peta diamond contains 22 carbon atoms: 10 are sp3 and 12 - by sp 2 atoms . In addition, the symmetry operators belonging to the Fm¯3m group reduce the number of independent atomic regions to three.
Since the network is made up of sp 2 (triple coordinated) and sp 3 (quadruple coordinated) carbon atoms , covalent bonds are classified into two groups. The calculated bond lengths related to sp 3 atoms are 1.563 Å for C1-C1 bonds and 1.520 Å for C2-C3. But the bond length (C3-C3) for sp 2 atoms is 1.349 Å, which confirms the presence of a double bond in sp 2 atoms .
As shown in 1a, penta diamond has large cubic "pores" with 3.664 Å edges, surrounded by a pentagonal covalent network. Consequently, it has a low mass density with a density of 2.26 g / cm 3 , like graphite, but 36% less than diamond.
The relative total energy of a penta diamond is 275 meV / atom, which corresponds to a classical diamond. But the total energy is higher than that of diamond, graphite and other hard carbon materials, albeit lower than that of C60, which is known to be a metastable zero-space carbon allotrope. The moderate total energy is explained by the structural distortion of the bond angles for sp 2 and sp 3 atoms .
Regarding sp 3 atoms : despite the fact that the C1 atom has an almost perfect sp3 hybridization with a bond angle θ212 = 109.4 °, the C2 atom has bond angles θ212 = 115.9 ° and θ323 = 101.9 °, which are wider and narrower than the corresponding bond angles of ideal sp 3 . Regarding sp 2 atoms : due to the pentagonal network, the bond angle related to C3 is θ232 = 133.4 ° and θ233 = 113.3 °, which is also larger and smaller than the corresponding bond angles for ideal sp 2 .
Moderate energy also increases the energy of formation (ΔE) of penta diamond upon direct copolymerization of C 7 H 8 and [5.5.5.5] -phenestran-tetraine:
spironadien + fenestratetraene = penta diamond + 12H 2 + ΔEThe calculated energy of formation is 0.31 eV / atom, reflecting the energy consumption for the formation of a covalent network of sp 2 and sp 3 carbon atoms with distorted bond angles.
Thus, it is expected that penta diamond will be synthesized using the Ullmann reaction for bromospiro [4.4] nona-2.7-diene and bromine [5.5.5.5] -phenestratetraene instead of their primary form.
Next, we studied the thermal stability of penta diamond by simulating molecular dynamics at a temperature of 4000 K. In order to understand what structural changes can occur, molecular dynamics calculations were performed with a constant temperature for an expanded atomic cell (88 carbon atoms) for 12 ps (picoseconds, 1 ps = 10-12 s) and for a simplified one (1x1x1) for 146 ps.
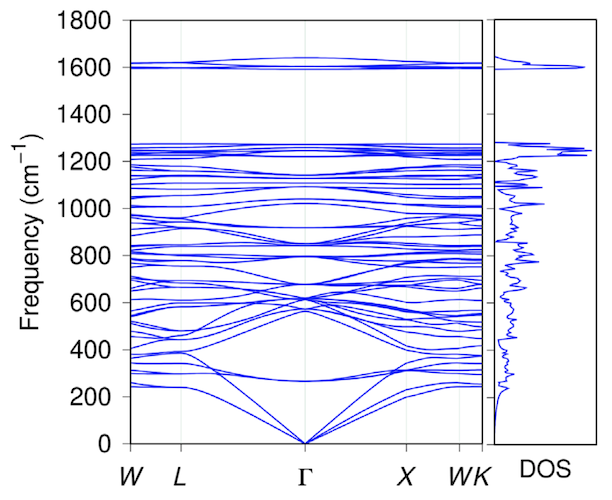
Image # 2
According to the simulation results after 14 ps at 4000 K, the penta diamond completely retains its original topology in both the complex and the simplified atomic cell (graph above).
Consequently, penta diamond is thermally and energetically stable if it is synthesized using the appropriate schemes proposed in this work.
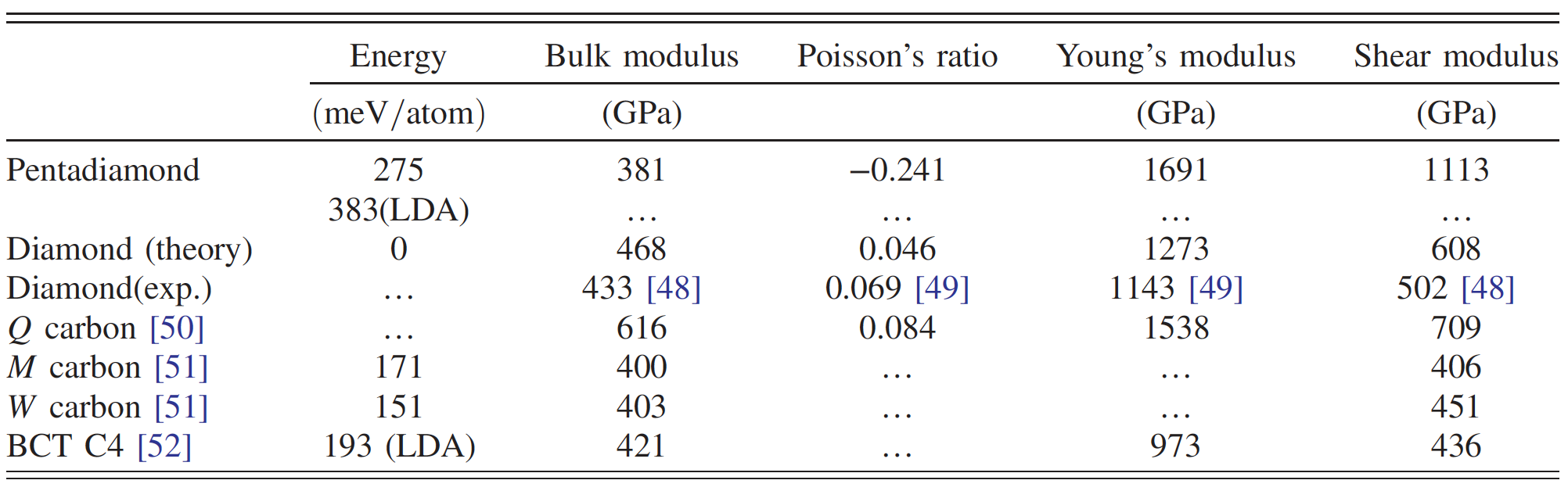
Comparison of the properties of penta diamond and other carbon allotropes.
The mechanical properties of penta diamond were studied by means of elastic constants cij, which are determined by evaluating the finite difference in total energy with respect to deformations. The calculated values of the elastic constants were: 1715.3 GPa for s 11 (= s 22 = s 33 ); −283.5 GPa for s12 (= s 13 = s 23 ) and 1187.5 GPa for s 44 (= s 55 = s 66 ).
It should also be noted that these indicators fully satisfy the Born stability criterion (with 11 - with 12 > 0, with 11 + 2c 12 > 0 and with 44 > 0), which additionally indicates the stability of penta diamond.
In the case of cubic symmetry, the bulk modulus of elasticity is calculated by the formula: B = (s 11 + 2s 12) / 3. As a result, B was equal to 381 GPa, which is more than 80% of the corresponding figure for diamond. This suggests that penta diamond is a potential candidate for solid carbon allotropes, although its density is quite low (like that of graphite).
For further investigation of the mechanical properties, Young's modulus of penta-diamond was calculated using the formula:
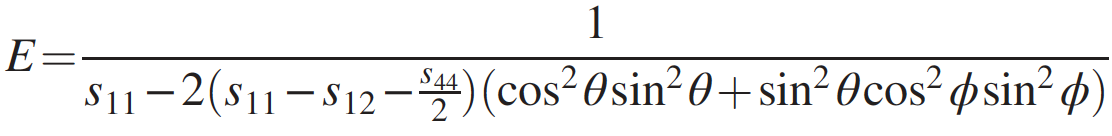
where θ and ϕ are the Euler angles * , s ij is the elastic compliance determined by cij with the relations between s 11 = [c 11 + c 12 ] / [(c 11 - c 12 ) (c 11 + 2c 12 )], s 12 = [-c 12 ] / [(c 11- c 12 ) (c 11 + 2c 12 )] and s 44 = 1 / c 44 .
Euler angles * - angles describing the rotation of an absolutely rigid body in three-dimensional space.
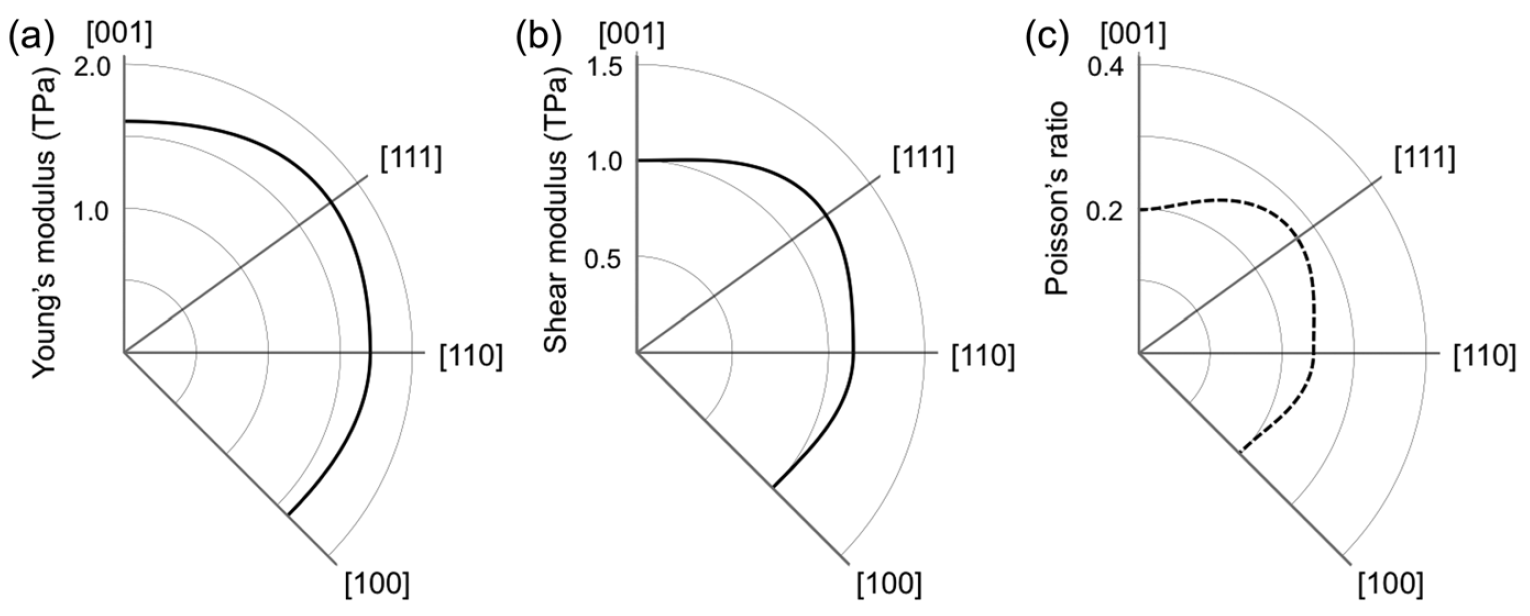
Image No. 3
Young's modulus of penta diamond is extremely high and exceeds 1.5 TPa for all directions ( 3a ). Knowing Young's modulus and bulk modulus of elasticity, it is possible to calculate the shear modulus ( 3b ), which also turned out to be quite high (1 TPa) for all directions.
Consequently, penta diamond can exhibit extreme rigidity relative to anisotropic structural deformations. The fact that Young's modulus and shear modulus are higher than that of other hard and superhard carbon allotropes (table above) also requires special attention.
Such high values of the above-described modules indicate that the penta diamond should have a negative Poisson's ratio. This statement was confirmed by calculations showing Poisson's ratio in the range from -0.20 to -0.28, depending on the directions of the grating ( 3c ). Such unique indicators lead to the fact that the speed of sound in penta diamond will also be quite high (28700 m / s versus 12000-18350 m / s for ordinary diamond).
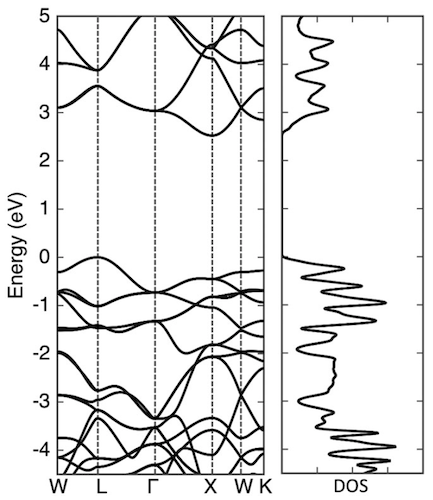
Image No. 4
The graph above shows the electronic structure and density of states of penta diamond, which is a semiconductor with an indirect band gap of 2.52 eV. The valence and conduction bands are located at points L and X, respectively. The highest branch of the valence band and the lowest branch of the conduction band have significant dispersion (1 eV or more).
Therefore, it is expected that penta diamond will have small effective masses at the edges of the bands: the calculated masses of electrons at point X are 0.98 and 0.67 (less than that of diamond) along the directions to the points Γ and W, respectively. But the situation with respect to the edge of the valence band is the opposite: the calculated masses of holes at the point L are 1.59 and 0.76 (greater than that of diamond) along the directions to the points W and Γ, respectively.
The moderate carrier mass and high density of states at the band edges suggest that penta diamond can have moderate carrier mobility for both an electron and a hole. The dispersion relation as well as the density of state show that penta diamond has a three-dimensional volumetric electronic structure, like a diamond, which reflects its three-dimensional covalent network with high symmetry.
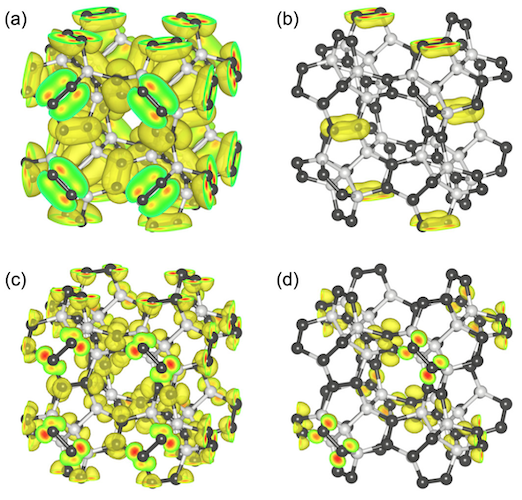
Image №5
For a better understanding of the electronic structure of penta diamond, scientists conducted a study of the wave function of the highest branch and the lowest branch of penta diamond at certain points of symmetry.
The wave functions of the higher branch of the valence band at the points L and Γ are distributed over the C3 atoms, which have the character of a π-bond, due to their sp 2-hybridization. The wave function of the lower branch of the conduction band at the points Γ and X is also distributed over the C3 atoms with an antibonding π-nature. Scientists note that both the valence state and the conduction state are not a pure π-state, but hybridized states containing a small amount of the σ-component. This means that the electronic states near and around the band edges are considered as π-electronic states of sp 2 C dimers , which are approximately 2.6 Å distant from its eight adjacent regions.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists .
Epilogue
In this study, scientists have advanced the theory that it is possible to create a carbon structure with better properties than diamond. The calculations performed during the study confirmed this conjecture.
By copolymerization of spiro [2.4] heptane-4.6-diene (C 7 H 8 ) and [5.5.5.5] -phenestrane-tetrain with Fm¯3m symmetry, a three-dimensional covalent pentagonal network of sp 2 and sp 3 carbon atoms can be synthesized . Scientists called their creation a penta diamond.
Most of the mechanical properties of penta diamond are superior to those of ordinary diamond known to us or other hard carbon allotropes. Curiously, penta diamond is harder than diamond, but its density is similar to that of graphite.
In the future, scientists intend to translate theory into practice. But even now, their work clearly demonstrates that the possibilities of modern science are truly endless, if, of course, you pay due attention to details, remember all the nuances of the laws of nature and not be afraid to experiment.
Friday off-top:
BBC , . ( , )
BBC , . ( , )
Off-top 2.0:
, , ? , , .. - …
, , ? , , .. - …
Thanks for your attention, stay curious and have a great weekend guys! :)
A bit of advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends cloud-based VPS for developers from $ 4.99 , a unique analog of entry-level servers that was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server? (options available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper at the Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to build the infrastructure of bldg. class using Dell R730xd E5-2650 v4 servers costing 9,000 euros per penny?