
, Fullstack- Python, . ? Python.
1926 , . , , « ». :
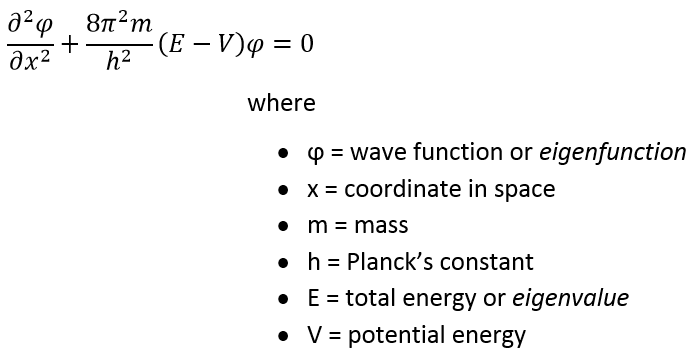
, :
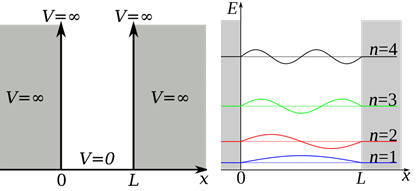
« » . - . (. . ), 0. , , . , . V , , , :
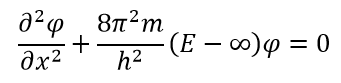
V , , , :
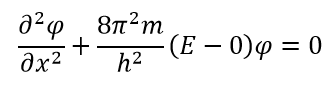
:
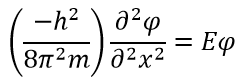
, , , E. :
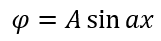
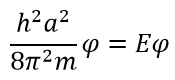
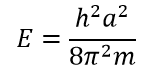
α A. , 0.
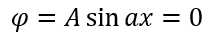
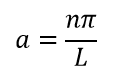
α:
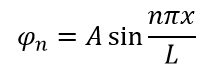
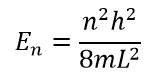
A. , - . , 1:
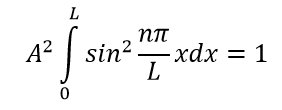
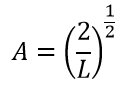
, :
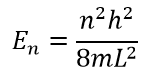
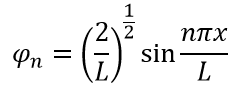
Python:
import matplotlib.pyplot as plt
import numpy as np
#Constants
h = 6.626e-34
m = 9.11e-31
#Values for L and x
x_list = np.linspace(0,1,100)
L = 1
def psi(n,L,x):
return np.sqrt(2/L)*np.sin(n*np.pi*x/L)
def psi_2(n,L,x):
return np.square(psi(n,L,x))
plt.figure(figsize=(15,10))
plt.suptitle("Wave Functions", fontsize=18)
for n in range(1,4):
#Empty lists for energy and psi wave
psi_2_list = []
psi_list = []
for x in x_list:
psi_2_list.append(psi_2(n,L,x))
psi_list.append(psi(n,L,x))
plt.subplot(3,2,2*n-1)
plt.plot(x_list, psi_list)
plt.xlabel("L", fontsize=13)
plt.ylabel("Ψ", fontsize=13)
plt.xticks(np.arange(0, 1, step=0.5))
plt.title("n="+str(n), fontsize=16)
plt.grid()
plt.subplot(3,2,2*n)
plt.plot(x_list, psi_2_list)
plt.xlabel("L", fontsize=13)
plt.ylabel("Ψ*Ψ", fontsize=13)
plt.xticks(np.arange(0, 1, step=0.5))
plt.title("n="+str(n), fontsize=16)
plt.grid()
plt.tight_layout(rect=[0, 0.03, 1, 0.95])
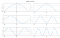
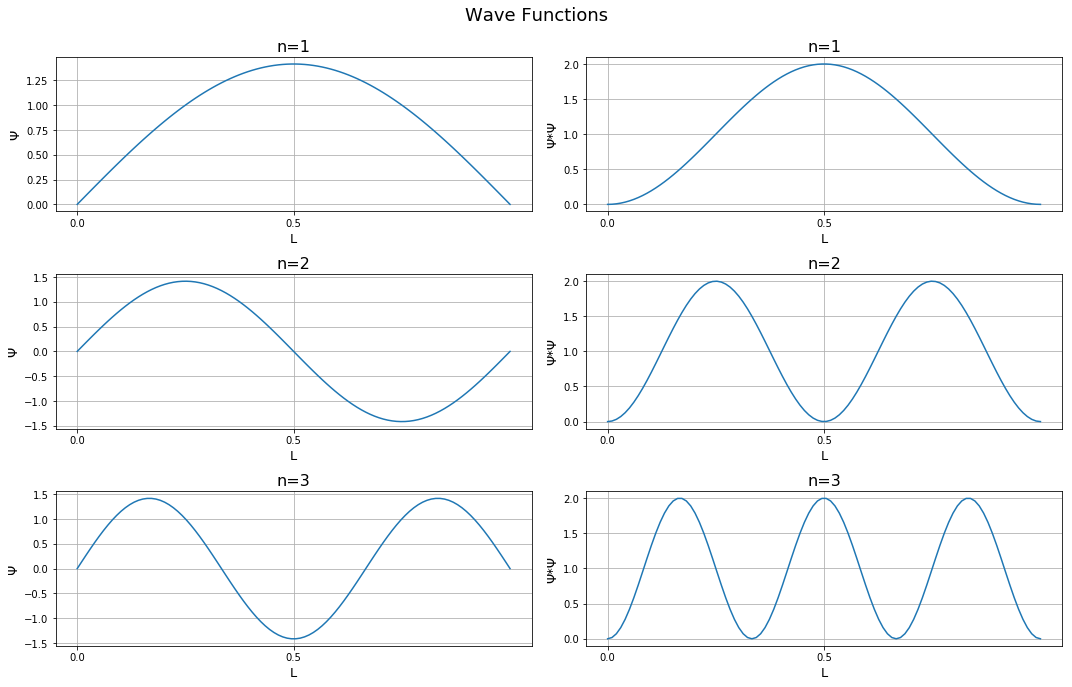
, , Ψ Ψ * Ψ . . . , . , n .
, , :
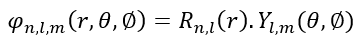
, . . R Y , . 1s:
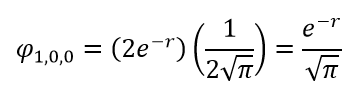
1s- , . .
import matplotlib.pyplot as plt
import numpy as np
#Probability of 1s
def prob_1s(x,y,z):
r=np.sqrt(np.square(x)+np.square(y)+np.square(z))
#Remember.. probability is psi squared!
return np.square(np.exp(-r)/np.sqrt(np.pi))
#Random coordinates
x=np.linspace(0,1,30)
y=np.linspace(0,1,30)
z=np.linspace(0,1,30)
elements = []
probability = []
for ix in x:
for iy in y:
for iz in z:
#Serialize into 1D object
elements.append(str((ix,iy,iz)))
probability.append(prob_1s(ix,iy,iz))
#Ensure sum of probability is 1
probability = probability/sum(probability)
#Getting electron coordinates based on probabiliy
coord = np.random.choice(elements, size=100000, replace=True, p=probability)
elem_mat = [i.split(',') for i in coord]
elem_mat = np.matrix(elem_mat)
x_coords = [float(i.item()[1:]) for i in elem_mat[:,0]]
y_coords = [float(i.item()) for i in elem_mat[:,1]]
z_coords = [float(i.item()[0:-1]) for i in elem_mat[:,2]]
#Plotting
fig = plt.figure(figsize=(10,10))
ax = fig.add_subplot(111, projection='3d')
ax.scatter(x_coords, y_coords, z_coords, alpha=0.05, s=2)
ax.set_title("Hydrogen 1s density")
plt.show()
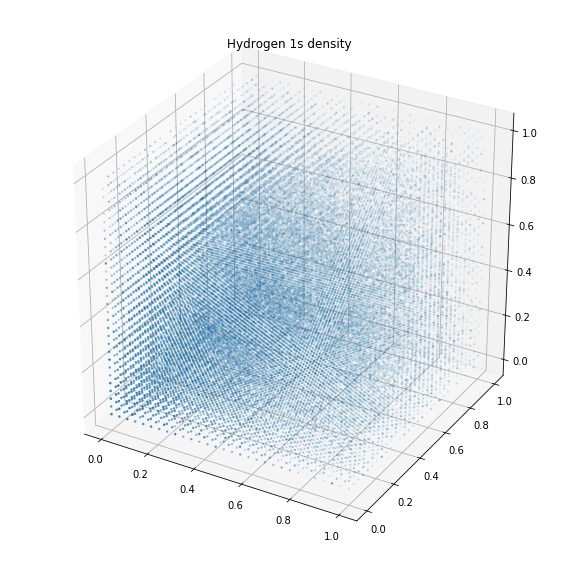
, . . , , 99 %. : s, p, d f.
, Python , . , , , , Python, Fullstack Python.
