22 healthy men kick a soccer ball for hours and nothing is done to it. A molecule of this shape must be very strong (I. V. Stankevich)
I was thinking about writing a long article on allotropy, inspired by the success of the post on the tin plague. But, nevertheless, this topic is too extensive and a professional chemist would have succeeded better. Therefore, I will limit myself to a story about my favorite allotropic modification of carbon - fullerenes. Fullerene is very popularized, but they mostly write the same thing about him. In 2010, when it was 25 years since the practical discovery of this molecule, they wrote a lot about it, and now they have already forgotten it - in my opinion, completely in vain.
Allotropy is a physicochemical phenomenon in which the atoms of a certain element can form molecules with very different configurations, or different crystal lattices. As a result, each allotropic modification has its own specific properties.
The bouquet of allotropic modifications in carbon is extremely heterogeneous. The most famous of them are diamond and graphite:

Diamond is the hardest substance of natural origin, and graphite flakes peel off easily, since the vertical bonds between them are very weak, and the horizontal ones are strong enough.
In addition to diamond and graphite, another allotropic modification of carbon is well known: soot (aka amorphous carbon):

, , . – , . – , ( ). – , .

, . , 1967 , – , , . . , , . 1960- , , . , , «» , , .
1960- , , . , .
, , , .
1985 , 10 60 70 . 60- , . 60 , C70 .

, C70 C60.

, , C540, :

, . 1967 .

, 1996 . , , . C60, .
, , . (C6 C6H6), . , , , , . :
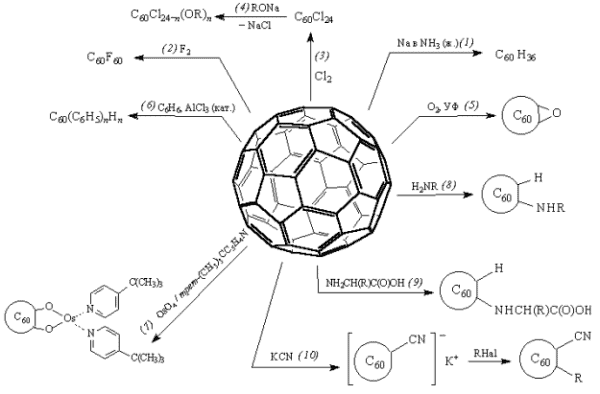
, , , , , , , . – . :

, . .
, . C60 5 . , . ( ). , M@C60, M – , .
, . : , 2011 ( : , ), 2018 . «ua-hosting.company» , . : :

, . , – . , .
1975 ( ), 1991 . – , , . – . – , , 2004 , 2010 . , , , . , . ( ) - , , . () , , . N+1:


: . . , , -- , .
2018 , , — , , . , 1880-. , . , , , . , .

, . , – , , . , « » - , « ». , «» «» . , . «» , – . « » . «», , , .
. C60 , – . , C60 . , , . , , .
On this, I run the risk of unduly blurring the subject of the article, since a more extensive technical excursion would require talking not so much about fullerenes as about graphene and its derivatives. Perhaps, if there is enough interest in this article, I will try to talk about graphene derivatives, but next time.
Technologies that would make it possible to stitch and build on carbon nanotubes of arbitrary length and shape from fullerenes - or, on the contrary, to lay out fullerenes and nanotubes in graphene layers - would become a major contribution to the history of the carbon age of human civilization.