
At first glance, it looks like this: you can take any substances, mix them in any combination, register them as cosmetics and start selling in pharmacies. Instead of safe fuflomycin, you can collect almost Alien saliva. And these drugs are in no way tested clinically. No double-blind, placebo-controlled studies (it's almost like having two surgeons reading an EKG).
The question is simple: why is that?
In short:
- For economic reasons.
- Due to the limited number of possible combinations and side effects.
- Because toxicology and microbiology are replacing part of the safety clinic.
- Because cosmetics, by definition, cannot have any effect on the inside of a person.
These are separate reasons, and now we will go through them separately.

1. Economic reasons
This is a near-European approach. You are expected to declare that your own research has shown the safety of the finished product. If a complaint comes up against you, you face a trial, terrible fines and a half-closure of the company. The local Rospotrebnadzor does not come to check the conformity of the certificate to reality immediately, but several years later. Maybe not at all. So the procedures there are very strict, but in fact they are of a notification nature. Another thing is that no one in this market usually plays with fire and does everything smoothly at once.
2. Limited possible combinations and side effects
Pharma and some medical devices are assembled in such a way that you can get anything at the end. Here was a case: they wanted to raise blood pressure - they got a group that for some reason stole samples of a test drug, and so on. No one knows how everything interacts there first with each other in the formula, and then with a set of substances inside a person. Therefore, many years of research are being done, when they monitor not only the direct action, but also the long-term side effects. And, as practice shows, it is not in vain.
In the case of cosmetics, clinical studies are carried out on the starting materials of the constructor. It is assumed that if you test each and then mix, you get a product that has the sum of the actions of the original components. Moreover, each substance has a special document, which tells what it can be mixed with and in what proportion. It is impossible to violate this regulation, therefore, it is impossible to synthesize the same sulfuric acid in the form of shampoo: there are no such sources.
The finished product is tested on animals or humans for toxicity, microbiological studies are done. But the medical effect is not tested.
By the way, packaging is also considered one of the active substances in this logic, so all tests are performed on the exact container that will go to end consumers. And each bottle has its own document, with which it can be mixed, and with which it cannot. And in what proportions. Therefore, only special bottles can be used for cosmetics: either profile bottles or food bottles.

3. Because there is toxicology
Still, in our club, gentlemen do not take their word for it, and at the end of the tests, toxicology is done. They take a mouse, open it, smear it, close it, watch how it will continue to run amusingly. By the time the laboratory technicians love it, they open it again, prepare it for research, and examine the resulting gruel. Not everything can be done on mice (especially if the product goes to the European market), so some of the tests are performed on human volunteers. But the ending there is different, without gruel. Naturally, physical and chemical indicators are also evaluated on this test, and not only the direct effect on the mouse: it is necessary to carry out rather complex calculations of what can go wrong, and in what dosages.
We are currently testing the latest lines of cosmetics on bull semen, kelp and cell colonies that roughly correspond to skin cells. Well, volunteering in beta, yes.
4. Because cosmetics do not heal
It is assumed that cosmetics do not get inside a person, that is, they do not have any systemic effect with any use. That is, yes, you can lose hair from the wrong shampoo, but (in theory) you are unlikely to rub the shampoo on yourself so deeply that it gets into the bloodstream.
How is this verified? According to the properties of the starting materials.
And this is where the interesting begins. The fact is that we have the same "Intensive Regeneration", which heals wounds faster than all the means known to us. She and I wanted to go to the ISS first aid kit, if that. Or demotene, a sulfur-based agent that removes demodex mites and helps treat demodicosis. No tick - no demodicosis. Why are they cosmetics and not medical devices with different testing protocols?
Because there is a commission that decides whether a cosmetics is a product or not. We had a gel, which a commission of eight people identified as a medicine: four in favor, three against, one abstained. That is, this is not a methodology or clear criteria, but simply value judgments and voting. For Intensive Regeneration, we were given permission to apply to the surface of the wounds, but we deliberately limited the indications to closed skin so as not to fly into the territory of medical supplies. For demoten, we have a letter of exemption - a document confirming that certification as a medical device is not needed. In fact, by the way, this means that when changing the packaging, the commission may vote differently, so any possible typo on such bottles will not be corrected for years.
“Intensive-regeneration” in the form of a cosmetic product was taken by a traumatologist and began to experience the healing of complex trophic ulcers. Preparing for publication with scientific work, we have had the results of a clinical study for a long time. But this research belongs to the "post-release" stage, that is, in fact, refers to the non-standard use of a standard tool and the actual expansion of indications. If we decide to produce a remedy for the healing of trophic ulcers, which will exactly coincide with the cosmetic product in composition, but differ in its purpose (and method of application), then there will be clinical trials.


How is the benefit proven?
The benefits are not proven in any way, the effectiveness of some indicator is proved. For example, if the hair should become thicker and silky, then you can conduct a study of the density and silkiness before and after, or you can protect yourself by analogy. To do this, you need to take another remedy with the desired effect, say: “We added the scent of orange, and the rest is the same,” prove that the flavoring does not affect the effectiveness in terms of reducing, and voila - you have a kind of analogue of a generic from the world of medicine!
By the way, we need to talk about generics separately. In the Russian Federation, the Law "On the Circulation of Medicines" dated 12.04.2010 No. 61-FZ is in force. For medical devices - Order of the Ministry of Health of the Russian Federation dated January 9, 2014 No. 2n "On approval of the Procedure for assessing the conformity of medical devices in the form of technical tests, toxicological studies, clinical trials for the purpose of state registration of medical devices." So when someone runs out of patent, that someone sells the formula, and the rest of them start producing it. Only here production in Belgium and China is slightly different both in the technical process (this is the most expensive and complex), and in the sources of components. For example, in Belgium, a component was synthesized in a vat with a colony of bacteria,and in China they decided to simply make a cadaveric extract of the same thing (in Moscow they do this with hyaluronic acid salts: we use European synthesized raw materials, and in the Russian Federation you can buy only cadaveric ones from processed bird ridges ... More precisely, you can buy any imported one, but they are produced domestically just like that). In order not to certify raw materials from scratch in five to seven years, a simplified procedure is used: it is necessary to prove that the properties of the new substance in the new plant are the same as those of the previous formula. This is proved by biodegradation and a number of other experiments. Depending on the type of product, research is also carried out, but this is far from a full range of analysis of the original. You need to achieve one hundred percent match.and in the Russian Federation you can buy only cadaveric stuff from recycled bird ridges ... More precisely, you can buy any imported one, but only the same is produced domestically). In order not to certify raw materials from scratch in five to seven years, a simplified procedure is used: it is necessary to prove that the properties of the new substance in the new plant are the same as those of the previous formula. This is proved by biodegradation and a number of other experiments. Depending on the type of product, research is also carried out, but this is far from a full range of analysis of the original. You need to achieve one hundred percent match.and in the Russian Federation you can buy only cadaverous from recycled bird ridges ... More precisely, you can buy any imported one, but only similar is produced inside the country). In order not to certify raw materials from scratch in five to seven years, a simplified procedure is used: it is necessary to prove that the properties of the new substance in the new plant are the same as those of the previous formula. This is proved by biodegradation and a number of other experiments. Depending on the type of product, research is also carried out, but this is far from a full range of analysis of the original. You need to achieve one hundred percent match.This is proved by biodegradation and a number of other experiments. Depending on the type of product, research is also carried out, but this is far from a full range of analysis of the original. You need to achieve one hundred percent match.This is proved by biodegradation and a number of other experiments. Depending on the type of product, research is also carried out, but this is far from a full range of analysis of the original. You need to achieve one hundred percent match.
According to a similar principle, it is possible to register medical devices in the Russian Federation, but one hundred percent compliance is no longer required. We find an analogue, we say that it has changed, we prove that this does not change the spectrum of research, we get a new certificate. That is, once upon a time in Russia, we registered one of the first gels for ultrasound through research.
The first cycle takes about five years, the next iterations take a year. We do not say that these are new gels, we conditionally release new releases of the old gel, iteratively updating it (from a medical point of view). During the tests, a comparison report is issued that the new product is functionally comparable. It is clear that in 10 steps of adding something small and removing something small, you can get a completely new product with a different formula, but that's what a commission is needed.
A couple more nuances
Registration of cosmetic products is done according to Regulation (EC) No 1223/2009 of the European Parliament and the Council of the European Union. The notification describes detailed information about the product, its safety, efficiency, and production. A safety report is generated for the cosmetic product "COSMETIC PRODUCT SAFETY REPORT" (CPSR) [ANNEX I No. 1223/2009], in which the safety margin is calculated. The calculation is carried out in accordance with the requirement of the Regulation (EU).
The data of the NOAEL indicator is used, the SED and MOS indicators are calculated by the formulas:
The Systemic exposure dose (SED) is defined as the dose of the component which is absorbed in a systemic way during the use or misuse of the product.
Dermal absorbtion of test substance reported in µg / cm2:
SED = DAa (µg / cm2) * 10-3 (mg / µg) * SSA (cm2) * F (day-1)
60 kg
Dermal absorbtion of test substance reported as a percentage of the amount of substance applied:
SED = A (mg / kg bw / day) * C (%) / 100 * Dp (%) / 100
SED (mg / kg bw / day) = Systemic Exposure Dosage
A (mg / kg bw / day) = Estimated daily exposure to a cosmetic product per kg body weight, based upon the amount applied and the frequency of application.
C (%) = the Concentration of the ingredient under study in the finished cosmetic product on the application site.
DAp (%) = Dermal Absorption expressed as a percentage of the test dose assumed to be applied in real-life conditions.
MOS = NOAEL / SED
In addition to the above, a toxicological dossier is collected for each ingredient.
The next moment. I don't trust government research because they have very light protocols. Two things are very important to me: not to withdraw the finished product from the market after the launch. And do not risk the reputation of your own plant with the purchased land under it and the trademark (in our case, this is the same thing). That is, all our tests are carried out much more seriously than those of state laboratories. It starts with us still before alpha: we try to destabilize the formula with temperature gradients, the most severe is freezing or heating in an oven at good temperatures for a long time. If the resistance of the medium, viscosity, transparency or some other physical indicator changes after the oven, the formula is considered unstable. We developed one of the remedies for four years: vitamin C dropped out,which behaved rather unpleasantly in the environment of the agent at high temperatures. Ordinary cosmetics on the Russian market can be released on the market in a month and a half (if you defend yourself according to literary sources and start producing before obtaining conclusions). Private laboratories often test on five to seven people and call it a clinic. At the same time, they do not compare the anamnesis (they took whoever they could, we had a group from 20 to 42 years old, and these are generally different types of skin). As a result, during the entire testing period, we have never received at least one negative result from private laboratories (accredited by the state). Not that it was bad, we feel pretty good before them, but still. And this is very bad, because we are most interested in them. Because commerce. In cosmetics, raw materials account for only a third of the unit cost.Everything else is the so-called invisible costs: production, tools of production, office (the same registration, that is, consulting doctors, conducting tests, developing research protocols, etc.), laboratories, tests.
We had a situation when a private laboratory, a state laboratory, did research on a group of less than 10 people, and we - on a group of about 100 people. At the same time, neither we, nor they have any of the side effects surfaced. But on post-studies with a much wider sample, it turned out that about 5% of the world's population turns red from our anti-couperose gel. This is an overreaction, a mild form of allergy. In general, it's okay, but you need to know about this, and you must also do allergy tests. We have introduced a warning about allergy tests everywhere - in all materials and instructions. This is an even more or less good situation.

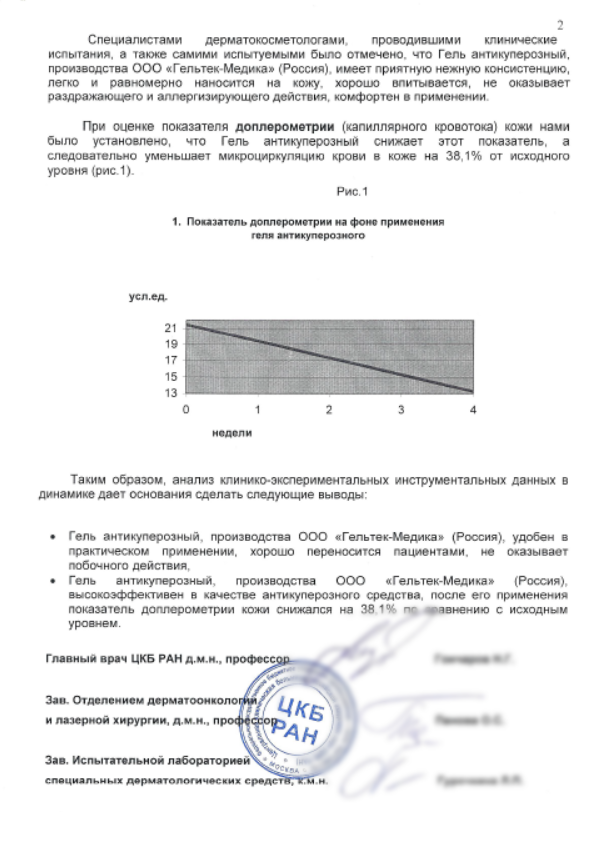
Worse was this: they made a tonic with aloe vera collagen. Our laboratory began with tests on 25 volunteers. Everything is fine with 23, two people did not like it: there was a feeling of contraction. This is a bad alpha, I immediately withdrew the formula from the development: this means that about 5-10% of the population will not like the tool. And the goal is for everyone to try, say: "Wow!" and advised friends (or rather, more often girlfriends). One unplanned reaction to such an alpha is an immediate reason to cut off the formula. Alpha can be 10 cycles, and 15 easily.
Checks in Europe
PIF or die. This is a set of product information data: there are studies and safety reports, evidence of effectiveness, all research protocols and all production processes, toxicology calculations, tolerances of all batches of components from the manufacturer, calculation of maximum damage, calculations for each of the components.
The PIF should be in the safe at the factory, and it should be shown immediately and without unnecessary dancing with a tambourine. Moreover, in Europe they check Russians quite viciously and without a chance to buy something. Natura Siberika built the plant in such a way that it was produced in Europe. A very good idea: the technical process, if very briefly, in our humble opinion, is the science-intensive transfusion from the supplied barrels into European bottles.
It costs cosmic money to make a PIF, because the same toxicology calculations are a huge trade secret. This is another reason why, even with research, no one really publishes it in cosmetics. At lectures for advanced training, the lectures themselves are not given, only notes, for example.
It cost 2,500 euros for the finished product (more precisely, 500 euros for the mutual fund plus 2,000 euros per year for the person in charge). Since we are not the richest company, but we have the best polymer chemists, in the first cycle they paid for the complete compilation of the PIF in a special laboratory, and then for almost three months they reversed it all in order to figure out what they were doing. There is also a lot of scary: if you are not a chemist with a higher education, you can get scared of all the tables. That is, a journalist, seeing them, will definitely rape a scientist. The general level of the consumer's intelligence does not correspond to the level of knowledge of the chemist, therefore, we believe that they are also not particularly shining: even in Europe they do not want to tell for months that the form is read as it seemed to you ... Now we are one of the few in Russia who does the PIF on its own. Perhaps the only ones.
Come blush for science!
As you can see, we need a very large program of our own tests: there is no need to make mistakes when entering the market. For 20 years we have been producing: we have our own production, equipment, all advertising is directly related to the plant (even new trademarks). We are launching the conclusion of a multi-year contract for the supply of funds to the United States (we are now going through FDA checks for joint production), we have invested in this a huge amount of money. Naturally, misfires are not needed. Therefore, now we are expanding the program of tests, in three years we have increased laboratory capacity by six times and are conducting extended tests (which no one in life does in cosmetics, but they do for individual components). And yes, we need volunteers to test cosmetics. This is beta, that is, the identification of rare side effects, all the main and terrible takes place on the alpha and pre-alpha on the researchers themselves.If you want to help as a test subject or if you know doctors who want to join our research program (this means early access and the ability to influence funds), write to epas@geltek-medica.ru.
If three years ago we made two products a year, now we are reaching a rate of about 10 new products a year. Because, finally, we have entered the foreign markets normally, and Russian cosmetics of our production are really needed there.