It is experimentally confirmed that an elementary particle must exceed the speed of light if it "tunnels" through a wall in a quantum-mechanical way.
As soon as the radical equations of quantum mechanics were discovered, physicists discovered one of the strangest phenomena allowed by this theory.
"Quantum tunneling" demonstrates how deeply elementary particles, such as electrons, differ from macroscopic objects. For example, throw a ball against a wall and it will bounce. Let it slide to the bottom of the hollow and it stays there. But in the first case, a particle can accidentally slip through the wall. A particle has a chance to "slip over a mountain and roll out of a hollow," as two physicists wrote in Nature in 1928 in one of the earliest characteristics of quantum tunneling.
Physicists quickly discovered that the ability of particles to tunnel through barriers allows many mysteries to be solved. This ability explains various chemical bonds, and radioactive decay, and thermonuclear fusion in the interior of the Sun, where hydrogen nuclei manage to overcome mutual repulsion and merge - as a result of which sunlight appears.
But the physicists were overcome by curiosity, at first mild, and then really painful. How long does it take for a particle to tunnel through the barrier?
The problem was that the answer was meaningless.
The first tentative estimates of the tunneling time were published in 1932. Perhaps, in private conversations, such assessments were made even earlier, but “when you get an answer that doesn't seem to make sense, you don't publish it,” notes Ephraim Steinberg , a physicist at the University of Toronto.
It wasn't until 1962 that Texas Instruments engineer Thomas Hartman wrote an article in which he openly accepted the shocking conclusions that flowed from mathematics.
, . , , . : , , , . , , .
, , , , .
“ ,” – .
, , . « , , , , ,” , - . 10 , , . .
, , , , .
, . .
, , Nature. « », , , .
“ – , , ,” , , Nature.
, - -, , . « ,” – .
. , , , , . , , .
, “ , [ ] ?” , “ , .”
?
, , .
, A B, , . .
. . – .
, , , . , - , , . « , », - , .
, , . , « », . , ( ? – . .). , . . B. , , B.
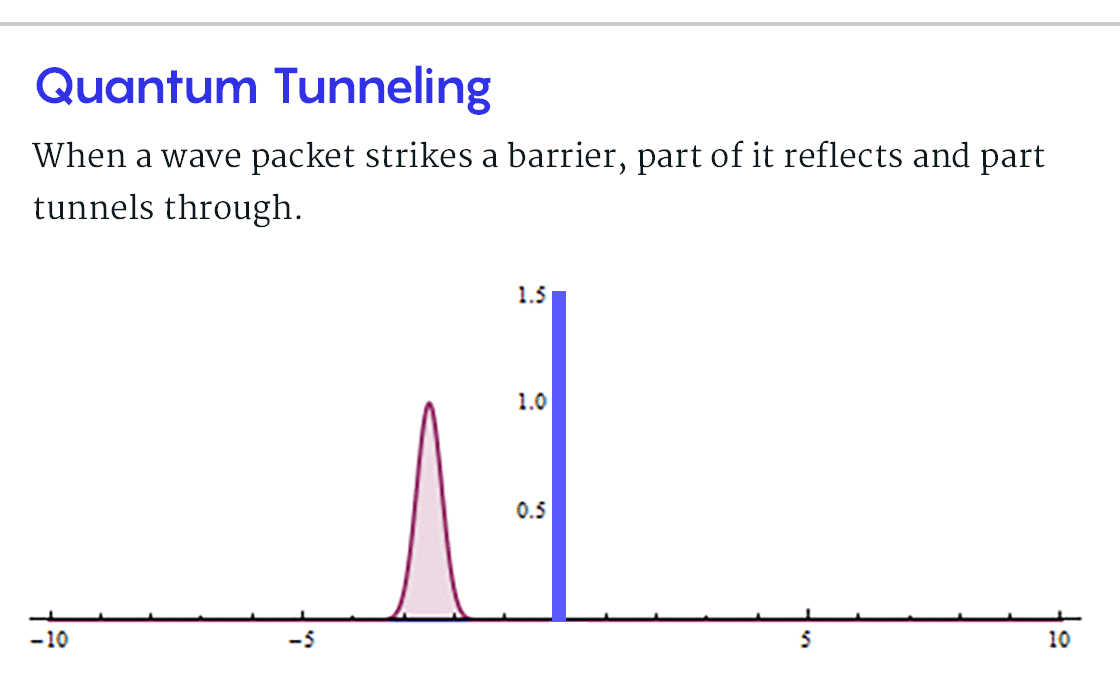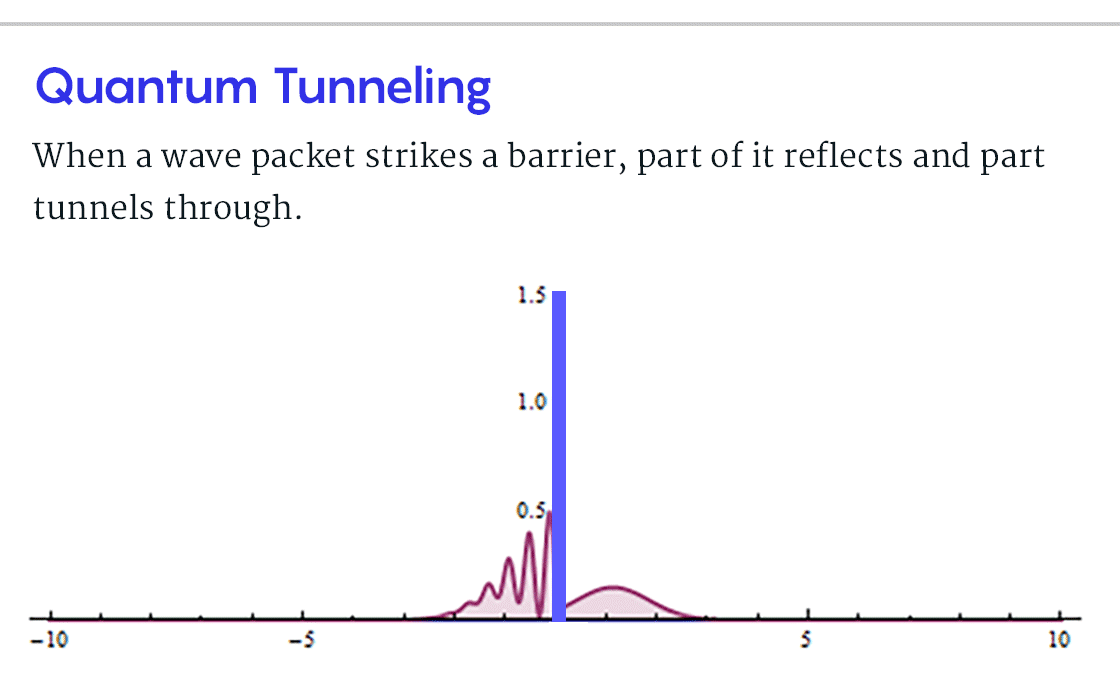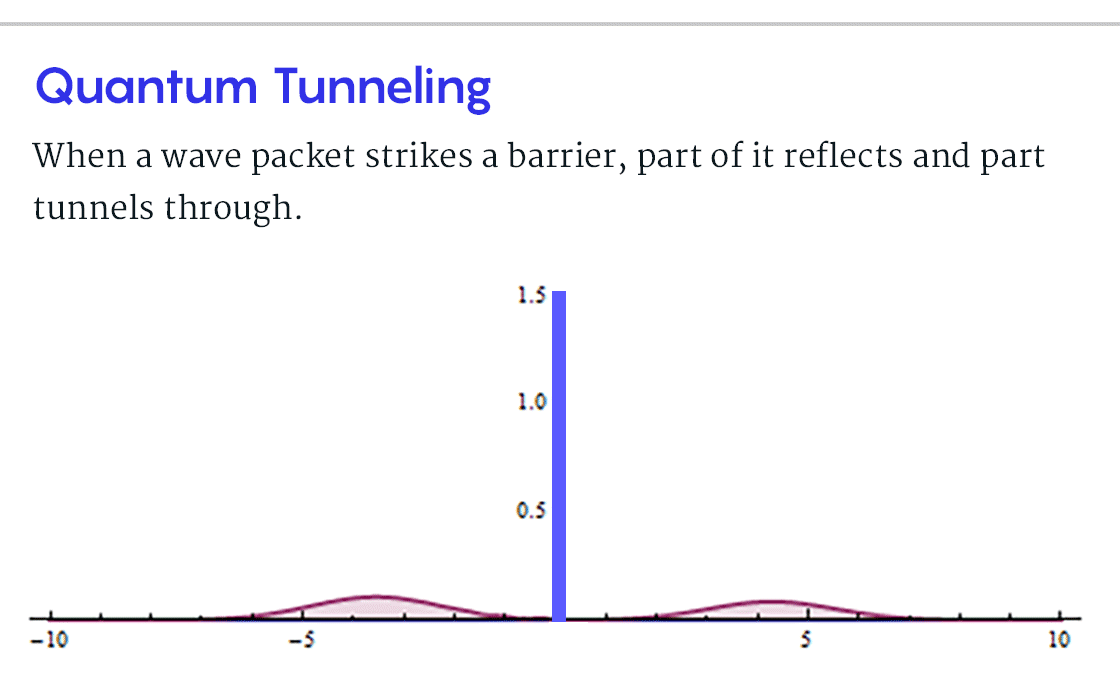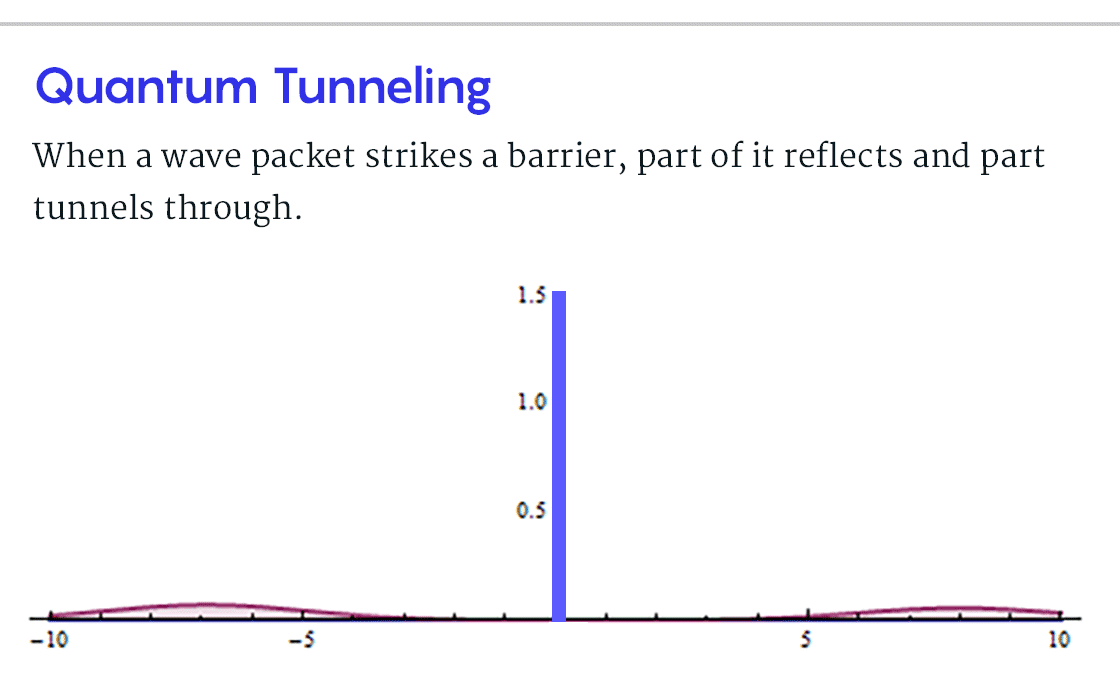
, B, , , ? , B, , , . . « » .
, -, , , A B, , - . – ?
, « » 1990-, , , . , , . «», . « : « ?», « ?»», - , - « , - ». , , , ( , , ). «».
. , ? «» .
( 1932 ) , , . A B , , . , .
, , . . ( A) ( B), , , , B, A. , , , , . B.
, . , , , , ( , ). .
, , 1960- , «» . «», , , . , .
, , . « , , , », - , Nature, - « , , ».
1980-, – , . . , , . , – , . , , , . , , , , . 50 , , .
, , 2019 , , . , — , .
, – . , , , , . , , , .
, , .
, , «». , . . 1897 , «», . , « ».
. , , . , . , «» «». , , , , – , .
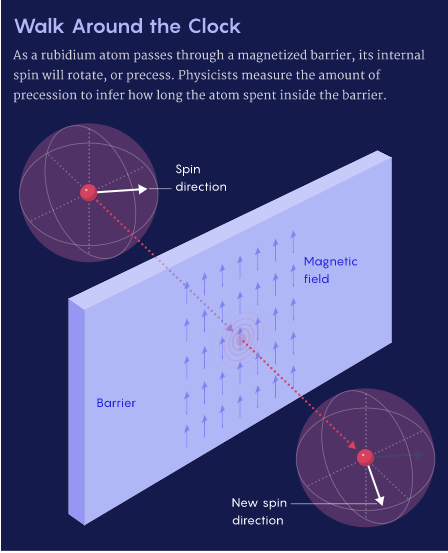
, 0,61 , , 1980-. , . , : , .
,
1907 , . , , . , . , , , , , . « », - .
, - , , . «, , », - , - « , ».
. , 2000- , . , , ( ). « , », - , - «, ».
, , . « », . « » , , . , .
, , , , . « , », - , , - « , . , ».
, New Journal of Physics, , : , , . .
- , , . , ? , , , , . , , .
, . « , . – ».
, . , « , , ». , , . , « ».
By probing many particles and averaging what exactly happens to them, the researchers depict in more detail what happens “inside the mountain”, which the pioneers of quantum mechanics could not even think of more than a century ago. From Steinberg's point of view, these developments suggest: despite all the oddities characteristic of quantum mechanics, "if you know where the particle ended up, you can determine in more detail what happened to it before that."
Inspired .