
I wonder how our research will end.
It seems that the past topic about GMO salmon has gone down quite well for Habrachiters. While we are preparing material on plant modification, I suggest looking at an equally interesting topic - genetic modification of Homo Sapiens. This is a very controversial and holivar topic, which is useless to ignore.
I will immediately give several theses that may seem controversial, but which somehow need to be solved in the scientific community:
- We are degenerating. Every year, weaker individuals survive, giving birth to children and burdening the gene pool. It needs to be fixed.
- Simple prohibitions won't help. If experiments are banned in Europe, then, for example, China will perform them.
- We will not stop at treating genetic diseases. Sooner or later, the military will join with their super-soldiers and those who want stronger and smarter children with blue eyes.
I will try to give an overview of the existing techniques of intervention in the genotype of people and at the same time we will analyze what it threatens us with.
What is gene therapy
Let's first define the terminology. Gene therapy is an intervention in the human genome with the aim of treating certain diseases. Moreover, the intervention is only in somatic cells. These are the cells that do not reproduce. We obviously do not need to fix any mutations and bug fixes in new generations. For now, at least.
Real animal experiments began around the 1980s, but then it was only timid steps. We didn't have any kind of beauty like DNA printers, expressing vectors for eukaryotes.
Where can we really intervene?
Plasmids
For a start, you can leave the cell nucleus, which contains the basic genetic information. You can use plasmids.
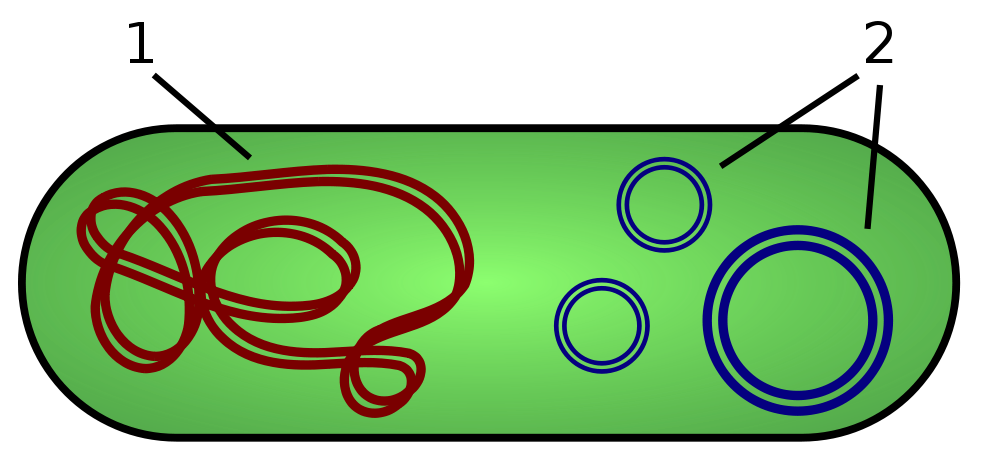
1) Chromosomal DNA of bacteria 2) Plasmids
Plasmids are almost exclusively bacterial. Sometimes found in primitive fungi and some plants. In fact, this is DNA, but folded into a ring and floating separately in the cytoplasm. That is, such a separate mechanism for storing genetic material. For bacteria, they are very important for transmitting positive mutations, for example, to “tell neighbors” about antibiotic protection options. The trick of plasmids when used in humans is that they fall apart on their own after some time after being introduced into the cell. And they also do not affect the main genetic apparatus of the cell. That is, the main firmware will not be affected.
Patching kernel
Next, we have the ability to directly edit the code snippet in the kernel. This is a more invasive procedure and already carries the risks of inaccurately inserting or cutting out a piece of DNA. But this is practically the only option to radically fix some serious genetic defect, since somatic cells will divide and transmit the patched version of DNA to their descendants.
Mitochondria
Mitochondria are like nuclear reactors in a cell. They consume fatty acids and glucose, and at the exit they stamp macroergs - substances with high binding energy. For example, ATP. It is a universal "fuel" for all active reactions, for example, for the operation of osmotic membrane pumps against a concentration gradient.
They have a peculiarity - their own genetic apparatus. Actually, they look like semi-autonomous symbionts for this very reason. Their genetic apparatus makes sense to rule with congenital mutations in mitochondrial DNA. Usually these are also very severe congenital syndromes.
Viral vector is like a syringe
The first key point is the development of delivery tools - viral vectors. The first such virus for mammals was developed in 1984. A murine retrovirus was used as a molecular syringe. He in itself was a very unpleasant tool, since with a high probability it provoked oncological diseases. Actually, its second name is murine leukemia virus (MLVs). In the future, the development switched to safer options.
What exactly is a viral vector? This is a specially modified virus that only works once. Like a syringe. To do this, he must meet several criteria:
Security
The viral vector should not be able to multiply spontaneously. For this they have a built-in kill-switch. Their genome is initially so damaged that they cannot reproduce autonomously. In a laboratory environment, they multiply in cell culture only in the presence of irreplaceable components and additional auxiliary viruses that produce part of the proteins required for assembly. That is, it multiplies in a laboratory culture flask, but in the body it works once, leaves the cell in a semi-assembled form and dies.
Low cytotoxicity
Many viruses are very cruel to the host cells. After the incubation phase, the cell dies of exhaustion and new virions scatter from its mortal remains in all directions. At the same time, there are viruses that do not particularly affect the normal physiology of the cells on which they parasitize. For example, adenoviruses.
Stability
Viruses must be genetically stable. For example, the flu won't work. He does not have any intelligible mechanisms of "parity" and he does not care that the hash after copying did not converge. This is his evolutionary strategy. The same coronaviruses, on the contrary, have mechanisms of control and repair, which gives greater stability of the genetic material. Although they are not used as a vector for other reasons. Thus, the task is to ensure that the virus in the process of its reproduction in bioreactors retains the same payload - the payload unchanged.
Cell type specificity
The virus must deliver its load not just anywhere, but exactly in a specific type of cells. This means that if we need to cure a disease associated with defective erythrocytes, then the virus must very accurately infect the multipotent precursor stem cells of blood cells. And at the same time do not hook, for example, muscle tissue.
What is already being done in terms of gene therapy
The first human genetic correction was carried out in 1990. 4-year-old Ashanti DeSilva received treatment for a severe genetic defect of complex combined immunodeficiency associated with a deficiency of the ADA enzyme. It should be noted that it was not the genome of stem cells that produce T-lymphocytes that were edited, but adult T-cells from her own donor blood. That is, she needed to repeat these procedures in the future. Then there was a series of successful experimental protocols for the treatment of SCID (Severe combined immunodeficiency) using a similar technique.
In 1999, an incident occurred that significantly slowed down research in this area. Jesse Gelsinger, suffered from a genetic liver disease due to which she was unable to detoxify ammonia. 4 days after the introduction of an adenoviral vector with a healthy copy of the gene, he died as a result of a hyperimmune response and multiple organ failure. Subsequently, the FDA concluded that there were numerous violations in the study protocol.
In 2006, the first reports of successful individual gene therapy protocols for the treatment of oncology appeared. Killer T cells are programmed for a specific type of tumor and then released into the patient's body. HIV therapy recognized
in 2011 in patient Gero Hütter in 2008. The method is not particularly applicable to a wide audience, as it requires the complete removal of one's bone marrow, and then implantation of corrected cells with a double delta-32 mutation that disables the CCR5 receptor.
By 2013, there were only five gene drugs licensed in the world. Three from oncology, glybera for the treatment of hereditary lipoprotein lipase deficiency and neovasculgen. The latter, by the way, was completely developed by us.
Neovasculgen
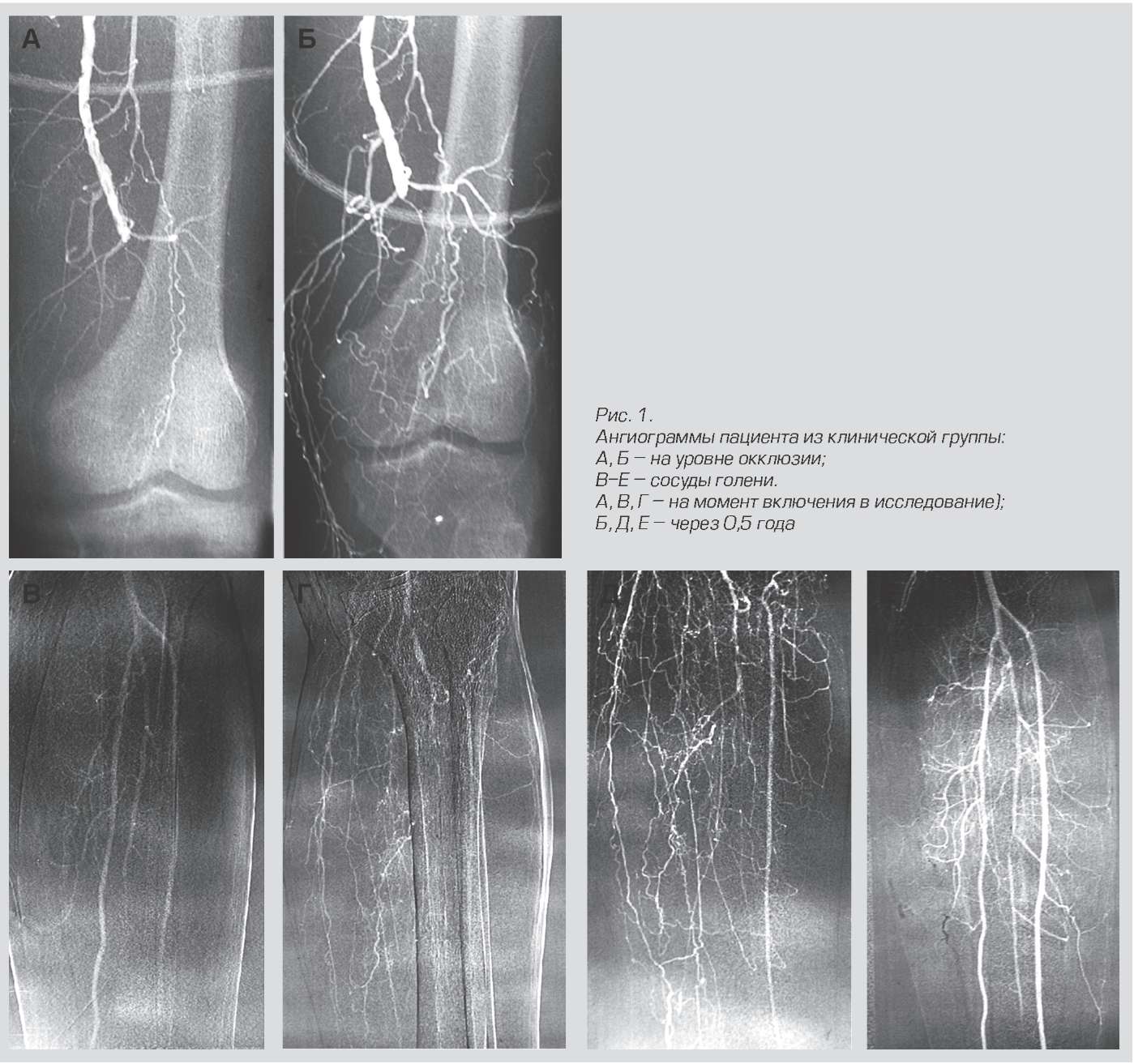
Results of treatment with Neovasculgen.
This drug belongs to the plasmid, that is, it does not edit its own genome of the cell, but only delivers plasmids into it that work for a limited time.
(), VEGF 165, (VEGF — Vascular Endothelial Growth Factor). , . , , .
Human Stem Cell Institute
The mechanism of action is interesting. The cells at the injection site begin to synthesize VEGF, a vascular growth factor. As a result, a new branched capillary network begins to grow in this area. This is critical for patients with chronic lower limb ischemia due to diabetes and atherosclerosis, for example. Before this drug, there were more amputations. Now it has also been tested for use in dentistry for the engraftment of implants. The gene preparation is mixed with bone material and sutured. As a result, the vessels grow rapidly and the necessary tissue is formed without rejection. The rabbits have already been accurately simulated during transplantation of skull fragments. People are next, as far as I know.
Zolgensma
Spinal muscular atrophy is a very grim disease, clinically somewhat similar to amyotrophic lateral sclerosis, which Hawking suffered, but has other causes. As a result of the development of the disease, paralysis progresses, which ends in death due to the inability to breathe.
Zolgensma is the first gene therapy drug for spinal muscular atrophy. Produced by AveXis (Novartis). A working copy of the SMN gene is injected using adeno-associated virus (AAV) serotype 9, AAV9, which is able to cross the blood-brain barrier and enter the patient's cells. One problem - it costs absolutely monstrous money. One injection costs more than $ 2.1 million (about 152 million rubles). But the results are amazing too. In humans, motor function is restored and the disease does not progress further. In theory, such expensive manipulations should be paid by the state. For individuals, the cost of treating rare diseases is simply prohibitive.
What's next?
Here is a very difficult question. We do accumulate defective genes in the population. Previously, a child with a heart defect would simply die - now they will save him and he will give offspring with this defect. Previously, many pregnancies were not saved - now pregnant women are pulled out with a minimum percentage of miscarriages and are completed with successful births. In fact, we are breaking the mechanisms of natural population culling. This is correct and humane, but something must be done with the accumulating mutations of the gene pool.
Either we will come to green cards and breeding permits, or we will learn how to reliably and safely correct genetic abnormalities. It would be cool to throw out myopia, systemic connective tissue dysplasia and a bunch of other birth defects. And finally, fix that stupid broken gene responsible for the synthesis of vitamin C from glucose, like all normal mammals.
Here only an unknown future awaits us further. Most alarming is the potential stratification of society, when the richest segments of the population will modify their children to be free of diabetes, atherosclerosis, and at the same time crush a little myostatin to make them look athletic without much effort. Cool. But it scares.
