The physical principles and possibilities of using superconductivity (including those already implemented) are described in abundance in the literature and on the Internet, so here we will limit ourselves only to a brief excursion into the essence of this phenomenon and the possibilities of its application, and then move on to the most interesting: what (breakthrough) discoveries in superconducting regions were completed literally in the past year.
Detailed and popular information about superconductivity is described in the book by Vitaly Ginzburg and Evgeny Andryushin, posted on the Elements website. A more popular presentation of the historical and practical aspects of superconductivity is in a very interesting material on Habré in the Toshiba blog. An article dated July 29, 2019, its indicators:

So, most substances can be attributed to conductors or dielectrics. Electric current is a sequence of electrons that penetrate at high speed through a material (primarily, conducting solids or liquids) from a source to a receiver. Any substance has some resistance index. Resistance is due to the movement of atoms in a substance, and these atoms capture some of the electrons from the flow, as they vibrate all the time, deviating from the base position. The higher the temperature, the more pronounced this phenomenon. But when a superconducting state is reached, any movement of atoms in a substance stops, and electrons penetrate through it without hindrance. Obviously, such a state must occur at very low temperatures, and that is why Kamerling-Oness discovered it for mercury, lead and tin at temperaturesclose to absolute zero, which is 0 K or -273.16 ° C. An arbitrarily weak electric current can persist in a superconducting substance indefinitely. Already in 1933 (Kamerling-Oness died in 1926) Walter Meissner and Robert Ochsenfeld discovered an equally striking property accompanying superconductivity: it turned out that the superconducting substance completely pushes out its own magnetic field. And this opens the way to such futuristic (then) things as magnetic resonance imaging andthat the superconducting substance completely pushes out its own magnetic field. And this opens the way to such futuristic (then) things as magnetic resonance imaging andthat the superconducting substance completely pushes out its own magnetic field. And this opens the way to such futuristic (then) things as magnetic resonance imaging and magnetic levitation , as well as the creation of thermonuclear reactors .
Here we note that Kamerling-Oness experimented with heavy metals, and also discovered the first superconducting alloy consisting of mercury, gold and lead. Accordingly, the search for substances that acquire superconducting properties at the highest possible temperature has become the primary task on the way to the practical application of superconductivity.

Metal, superconducting transition temperature and year of discovery. Source of illustration
So, the scientific search in the field of high-temperature superconductors has gradually moved from heavy metals to transition metals, alloys, intermetallic compounds and non-metals. Copper compounds (cuprates) and compounds with the participation of rare and rare-earth metals (samarium, yttrium) turned out to be especially promising.
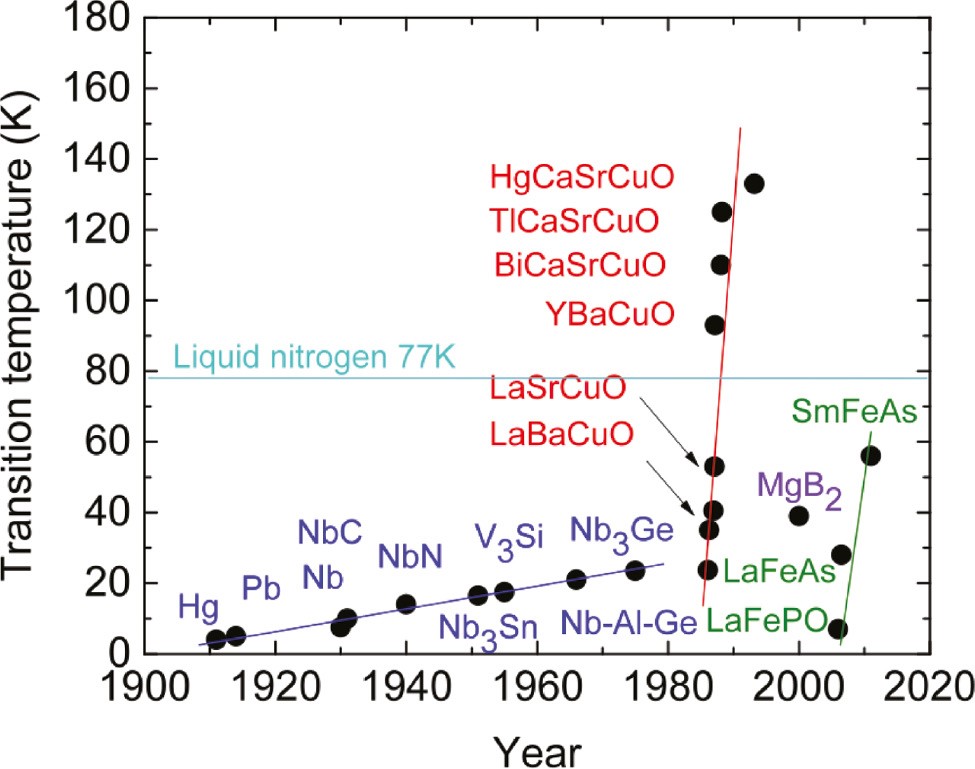
Source (October 2019)
As is clear from this graph, horizontally is the year of the discovery of superconducting properties in a substance, and vertically - the temperature of the transition to the superconducting state. Metals, compounds of metals with semi-metals and non-metals are indicated in blue. In this category, attention should be paid to niobium (Nb), whose compounds allowed for the first time to raise the superconductivity threshold to the region of 20 K. Cuprates are indicated in red, the most famous of which is, perhaps, YBaCuO (yttrium, barium, copper, oxygen) - the first compound , acquiring superconducting properties above the boiling point of liquid nitrogen.
Compounds of lanthanides (lanthanum La and samarium Sm) with iron (Fe) and elements of the nitrogen group (P, As) are shown in green - which is also logical, given that the superconductivity of niobium nitride was investigated back in 1940.
YBaCuO is so important in the context of this article that an image of its crystal structure, plus a detailed description of this structure, will now be given.
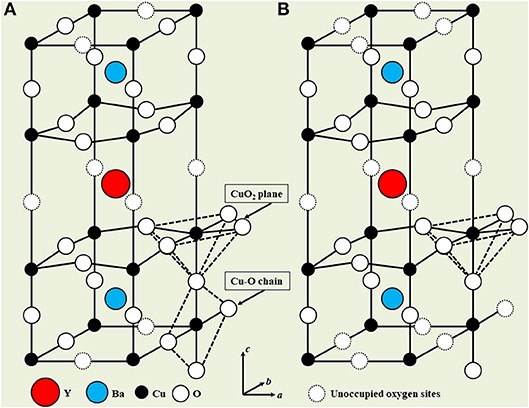
The crystal structure of YBa 2 Cu 3 O 7 −δ for (A) δ = 0 (YBa 2 Cu 3 O 7 ), in which all oxygen positions in the basal planes along the b axis are occupied, and for (B) δ = 1 (YBa 2 Cu 3 O 6) when all these positions are not occupied. An intermediate degree of filling with oxygen is achieved when such a sample is quenched in an oxygen atmosphere. The crystal structure is quadrangular for δ ≥ 0.6 and orthorhombic for δ <0.6.
The crystal structure of YBCO structure is complicated variation perovskite shown in Figure 4. As is clear from the figure, the unit cell is composed of YBCO cube YCuO 3 to the adjacent top and bottom cubes BaCuO 3, but at the same time some oxygen positions remain empty. Oxygen positions located in the same horizontal plane as the yttrium atom are never filled, due to which the existing oxygen atoms are slightly shifted towards the yttrium atom. The orthorhombic phase of YBa 2 Cu 3 O 7-δ has the following lattice parameters: a = 0.382 nm, b = 0.388 nm and c = 1.168 nm, when the δ value is very small. The oxygen content in YBCO determines its crystal structure and the frequency of holes in the CuO2 planes. At δ = 1, the compound (YBa 2 Cu 3 O 6) . δ = 0,4 , Y-Ba-Cu-O . Tc 92 K δ ≈ 0,06, , , . , δ < 0,06 Tc , , CuO2 .The formation of the quadrangular phase is observed at temperatures in the range of 700–900 ° C, and the orthorhombic phase is formed when the quadrangular phase is slowly cooled in an oxygen atmosphere to a temperature of about 550 ° C. During the transition from the quadrangular to the orthorhombic phase, many different twin domains are formed, since the stress is removed from the substance. In the quadrangular phase, oxygen atoms randomly occupy about half of the places allotted to them in the basal planes, during which they line up in the b-direction into Cu-O chains arising in the orthorhombic phase. Because of this, in the orthorhombic phase, positions for oxygen atoms are vacated in the a direction, which subsequently leads to a slight compression of the unit cell in such a way that a <b. Contribution to superconductivity is made as planes CuO 2 and CuO chains present in the orthorhombic phase.
The most important portion of the previous passage is in bold. Indeed, superconductivity and magnetic field repulsion are associated not only and not so much with the temperature of the material as with the atomic structure of its lattice. YBCO can be as well as a superconductor and an insulator; its properties depend on the positions of oxygen atoms in the crystal lattice.
Similar phenomena make it possible to achieve superconductivity in planar graphene layers located as close to each other as possible. When you rotate one graphene layer relative to another by the so-called " magic angle"(About 1.1 degrees) superconductivity appears; however, at very low temperatures, of the order of –269 ° C. More details about the superconducting properties of graphene are described in the material " Superconductor from flat graphene. Study of flat zones "on Habré.

Thus, a promising way to search for superconducting substances leads to studies of exotic compounds of metals with non-metals. As soon became convinced, metal hydrides go into a superconducting state at even higher temperatures than nitrides. At the same time, we note that it is possible to raise the temperature of such a transition not only by an ingenious selection of joints, but also by increasing the pressure.
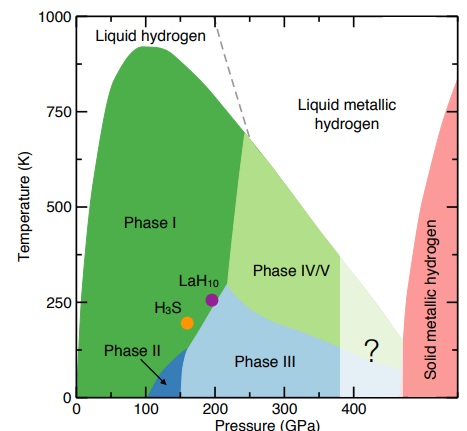
Until about 2015, cuprates undoubtedly dominated the leadership of increasingly high-temperature superconductors, and HgBa 2 CuO 4 + δ , (mercury-barium-copper-oxygen) synthesized in 1993, was the absolute record holder, passing into a superconducting state at a temperature of 164 K or - 109 ° C. But in 2015 it was discovered that at a temperature of 203 K (only -70 ° C), hydrogen sulfide H 2 S goes into a superconducting state ; however, such a transition requires a pressure of 1.5 million atmospheres, which practically excludes the possibility of using hydrogen sulfide as a superconductor. Nevertheless, this discovery gave rise to the search for superconductivity in hydrides.
In May 2019, the superconductivity of lanthanum hydride (LaH 10 ) was confirmed at a temperature of -23 ° C - at this temperature and pressure of about 2 million atmospheres, lanthanum hydride got rid of its magnetic field. In November 2019, thorium hydride ThH 10 was obtained , in which superconductivity occurs at a temperature of –112 ° C and 1.7 million atmospheres. Skoltech specialists Artem Oganov and Ivan Troyan play a key role in this achievement.
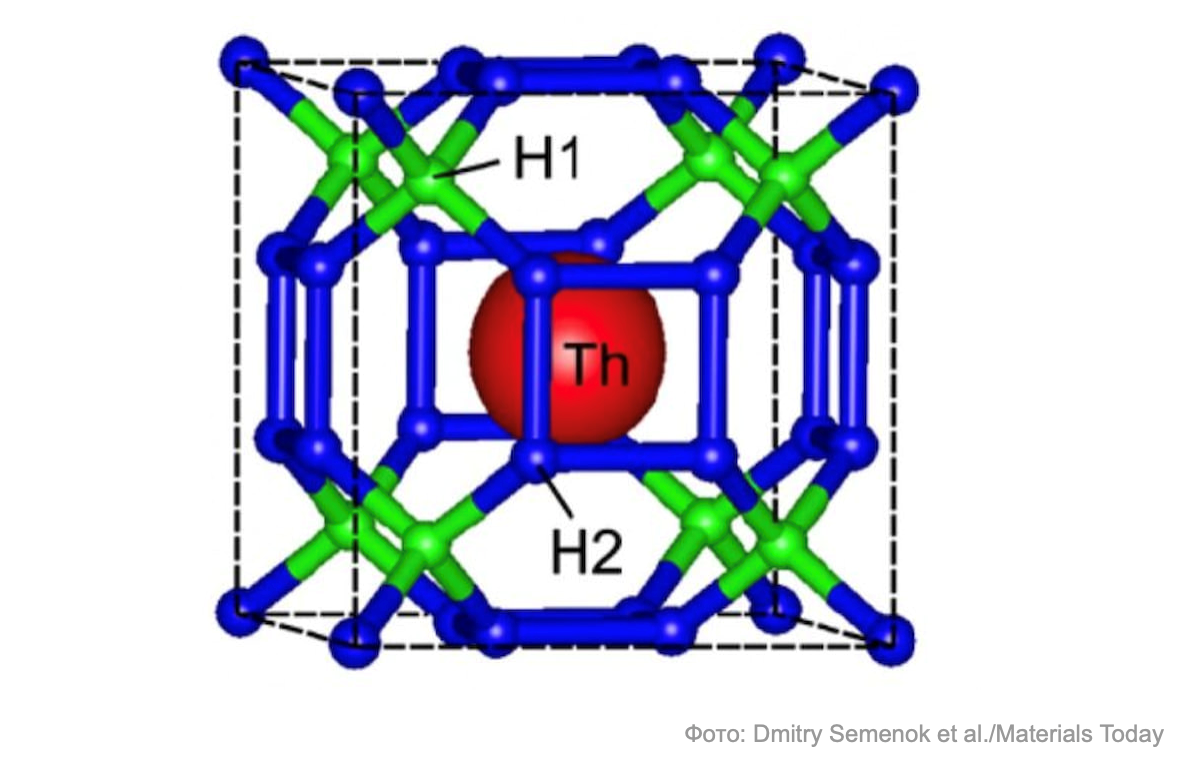
Finally, in October 2020, the University of Rochester managed to achieve superconductivity in carbonaceous sulfur hydride at a pressure of about 2.6 million atmospheres and at temperatures close to room temperature: 15 degrees Celsius.
So, there are two ways in which science is approaching relatively cheap and feasible high-temperature superconductivity:
Experimenting with cuprates at atmospheric pressure, gradually achieving an increase in Tc to moderate freezing, to about 200 K (-73 ° C).
We are experimenting with hydrides, which have already made it possible to obtain superconductivity at room temperature and are trying to reduce the pressure from millions of atmospheres to an acceptable one.
Of course, it is necessary to mention the third way, namely the use of boron compounds. In the right corner of the chronological table above is magnesium diboride MgB 2 .
It is obtained by sintering simple substances (boron and magnesium) and is already used in the construction of tomographs as a replacement for niobium-titanium alloys. The critical temperature of this substance is –39 K, that is, it is much higher than that of superconducting niobium compounds. Experiments with boron-based superconductors continue (let us reiterate that this class of substances achieves superconductivity at normal atmospheric pressure), and one of the most promising boron-containing materials is BSiC 2 , an article about which has been publishedin March 2020. According to theoretical calculations, it should reach T c at a temperature of about 73.6 K, and its related, more stable compound BC 3 at temperatures of about 40 K.
There are cautious assumptions, according to which pure metallic hydrogen can be an ideal superconductor operating at room temperature. Moreover, according to the scheme given in the article "Superconducting hydrides under pressure" published on September 26, 2019, solid metallic hydrogen could maintain superconducting properties up to temperatures above 750 K, that is, up to almost 500 degrees Celsius. On the other hand, this would require colossal pressure - more than 400 GPa.
One approach, presumably allowing to reduce pressure to obtain superconductivity with the participation of hydrogen - to experiment with hydrocarbon compounds that will be maximally saturated with hydrogen atoms, and carbon will provide strong electronic bonds, potentially allowing the material to remain intact even when pressure is released. Nevertheless, experiments with compounds including carbon, hydrogen and sulfur do not yet give the desired result, probably because quantum mechanical effects between atoms, which have not yet been taken into account, come into play.
At this point, the review could have ended with a timid "well, let's get metallic hydrogen - then we'll talk", but we'll end it differently.
Artem Oganov and Ivan Troyan, who were mentioned above in connection with their discovered superconductivity of thorium hydride, as well as graduate student Dmitry Semyonok from Skoltech and Alexander Kvashnin from MIPT developed the evolutionary USPEX algorithm. This algorithm makes it possible to predict the achievement of temperature T c in a particular crystal structure, depending on the position of its constituent elements in the periodic table. A detailed overview of the USPEX algorithm is provided here . At present, it is planned to train a neural network to recognize these dependencies and look for connections that will allow to bring the pressure providing high-temperature superconductivity to acceptable values.
It remains to be hoped that the success of this team is very close.