On November 9, the American pharmaceutical giant - Pfizer announced the successful completion of the third phase of clinical trials of the BNT162b2 vaccine against the new coronavirus, and the results obtained are 90% effective.
It is curious that just 2 days after the Pfizer press release, the website of the Russian Sputnik V vaccine (named after the prototype ship that sent the first man into space) published a message about its 92% effectiveness.
In this article, we will compare these two vaccines, analyze how they work, and also talk about why the world scientific community was quite critical of the Russian vaccine.
Content:
Comparison of preliminary results of the third phase
How immunity to viruses appears
Types of modern vaccines
How Sputnik V vaccine works
What is the difference between a vaccine and Pfizer
Outcome
Comparison of preliminary results of the third phase of clinical trials
, - BNT162b2, Pfizer BioNTech, 43 , 90% .
, 94 COVID-19 , , 90% , . — 7 . 38 . — 14 , [1].
« V» 92% , COVID-19 16 21 , 20 [2].
« V» , 3- [3], , , 20 . , [4].

, . , , "" .
, — ( ) [5].
, , , — . , - — , - .
: B- ( Bone marrow — ) - ( Thymus — ). , - , , — - . - () - (-), — , .
- -, , — , - , , - , , , , .
— , , .
:
, , . , , . :
— , — — . , , MMR — , . .
— — , , — , , [6].
— , . , , — , , [7].
« V»
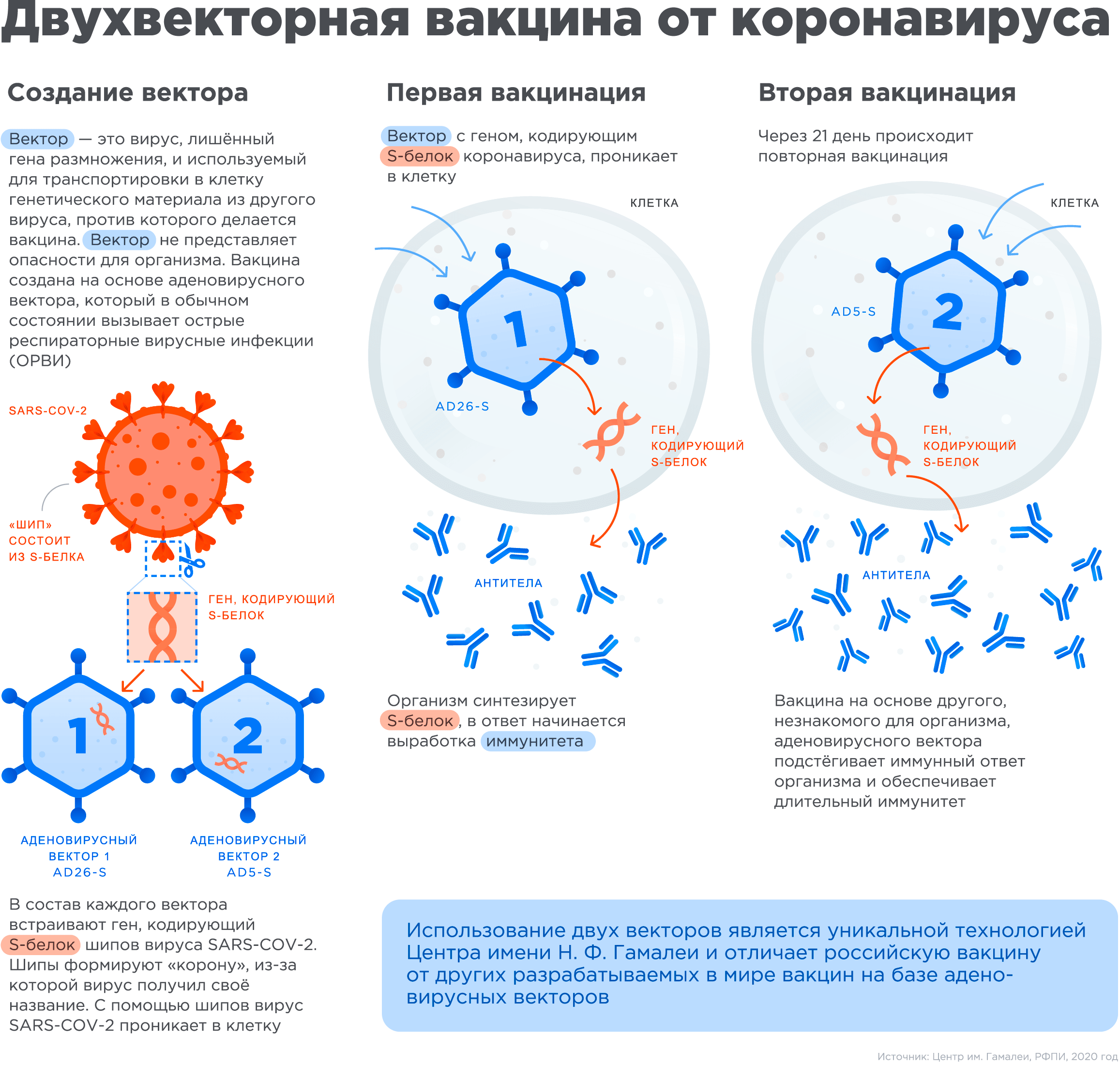
«--» « V» . , : — -, , , , .
« V», . . . Sars-COV-2, (spike protein), .
: , , , - , -, [8].
Pfizer
BNT162b2 Pfizer BioNTech , “” « V» .
BioNTech , , () , - [9].
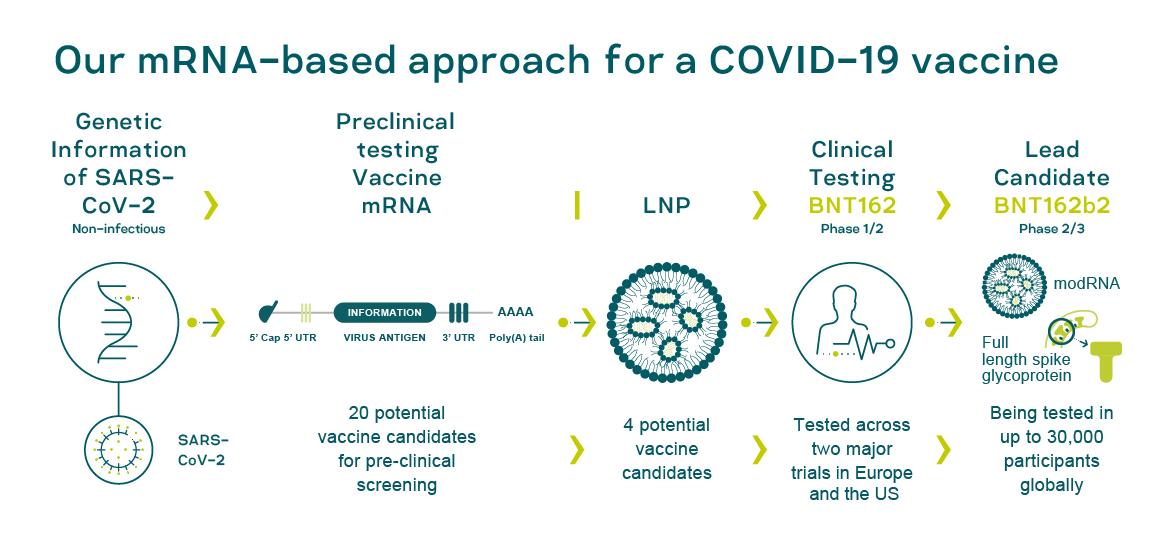
BioNTech . Sars-COV-2 , « V» BNT162b2 — .
BNT162b2 « V» , .
. « V» [3] [4].
:
, , , .
, , « », , .
1 https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
2 https://sputnikvaccine.com/rus/newsroom/pressreleases/effektivnost-vaktsiny-sputnik-v-protiv-koronavirusa-sostavila-92-v-khode-pervogo-promezhutochnogo-an/
3 https://www.nature.com/articles/d41586-020-02386-2
4 https://www.nature.com/articles/d41586-020-03209-0
5 https://www.ncbi.nlm.nih.gov/books/NBK27140/#A449 6 https://www.niaid.nih.gov/research/vaccine-adjuvants
7 https://www.niaid.nih.gov/research/vaccine-types
8 https://www.gamaleya.org/research/vaktsina-protiv-covid-19/
9 https://biontech.de/covid-19