
Human life by the standards of the Universe is just an instant, and by the standards of a fly-by-night it is an eternity. For us, the assessment of the duration of our own life path is complicated by the events that occur in the process, the people we meet and with whom we part, the emotions we experience. After all, the more complex the brain of a creature, the more complex its self-awareness. However, any path is determined not only by its richness, but also by the fact that it ends sooner or later. For many centuries, people have been trying to answer the question of what lies beyond the bounds of life, and no one has a clear categorical answer. Nevertheless, "before" remains no less mysterious and mysterious than "after". Scientists from the American Museum of Natural History (New York, USA) conducted a study,which describes a possible variant of the origin of organic molecules billions of years ago. What could serve as the beginning of life on the planet where it happened, and how can this knowledge help in understanding our world here and now? The answers to these questions are hidden in the report of scientists. Go.
Research basis
As mentioned earlier, it is not known for certain what awaits a person or any other living organism after his body loses its vitality. This question is asked by physicists, biologists, theologians and philosophers. They all have answers, each of which has the right to exist, but these are only theories that have not yet been empirically confirmed for obvious reasons.
As for what happened before everything began, this question is just as complex and ambiguous. The big bang theory gave us the idea of the origin of the universe, but questions remain about what came before it. Darwin's evolutionary theory helped to understand how the species on our planet are interconnected, how they evolved, how one was transformed into another. But even here questions arise: what or who was the first, why life was born, under what circumstances, was this event by chance or was it someone's great plan. Questions for centuries, not otherwise.
Nevertheless, the knowledge possessed by modern man can serve as a tool in building the chain of events that led to the birth of life. We know that hydrogen, nitrogen and oxygen are fundamental elements in the origin and maintenance of life. In modern life, most organic molecules are formed by the reduction of carbon dioxide (CO 2 ) through several “carbon fixation” pathways (for example, photosynthesis in plants). But most of these pathways either require energy from the cell, or they appeared relatively late. The question arises - what happened before?
According to scientists, one of the ways for the formation of organic matter could be the reduction of CO 2 with the help of H 2 . Geological studies show that CO2 was found in relatively high concentrations in the ocean during the Catarchean * , while H 2 was the product of multiple processes in the earth's crust and was released outward by hydrothermal vents.
Katarchei * is a geological eon (period of time) that lasted the first 600 million years of the existence of the Earth.Consequently, at the junction of two environments (the ocean and the Earth's crust), a reaction arose between two dissolved gases, which led to the formation of hydrocarbons, which subsequently played an important role in the transition from geochemistry to biochemistry.
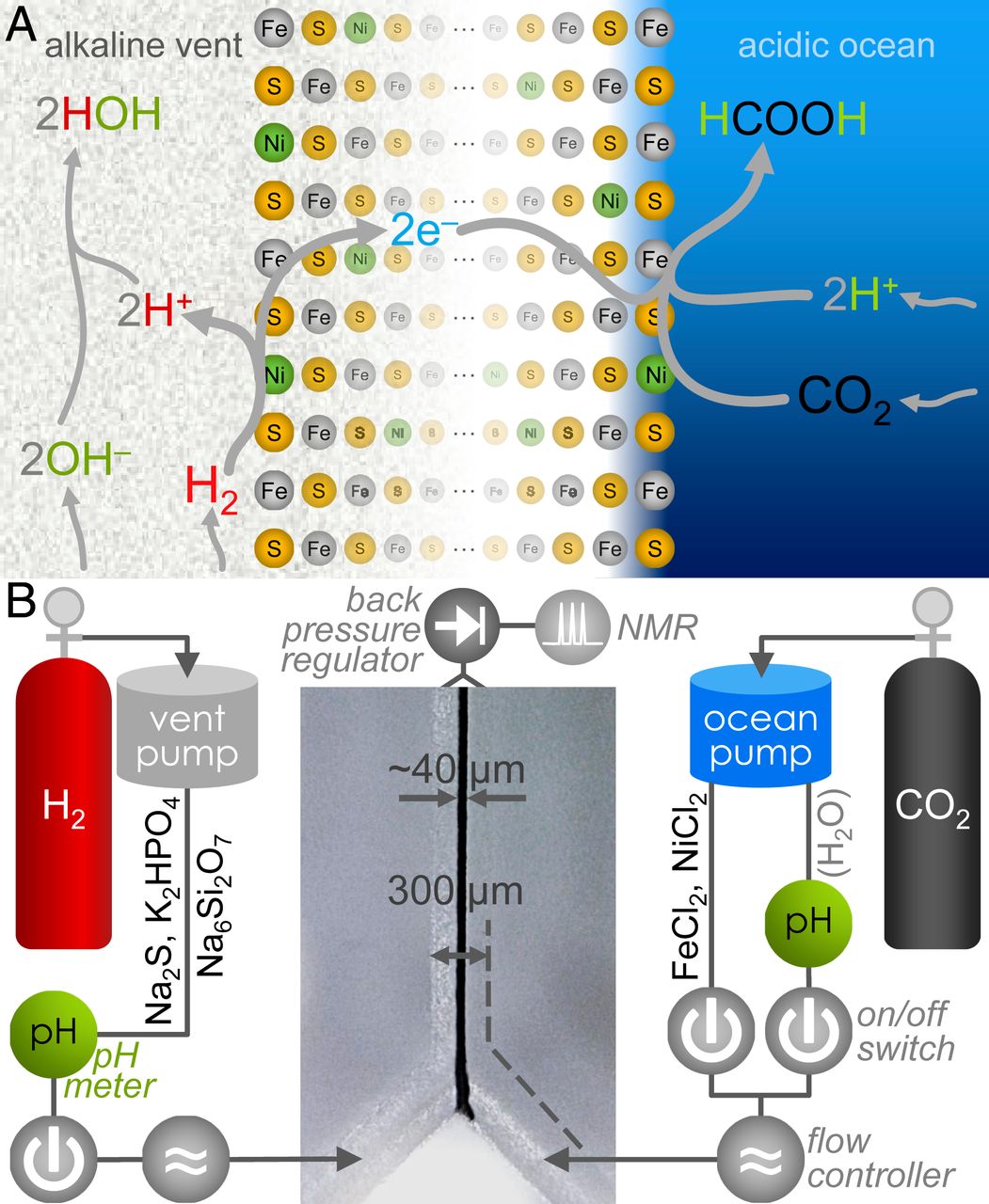
Image №1
Under standard conditions (1 at, 25 ° C, pH 7), the reaction between CO 2 and H 2 with the formation of formate (HCOO - ) is thermodynamically unfavorable with ΔG 0 ' = + 3.5 kJ / mol. However, in ancient alkaline springs ( 1A ), H 2 was present in OH - rich waters of a hydrothermal source, which promoted its oxidation into water. Moreover, CO 2would be dissolved in a relatively acidic ocean, which facilitated protonation in its reduction to HCOO - .
With the help of Fe (Ni) S minerals deposited at the interface between the ocean and the crust, a pH gradient of more than three units should have been sufficient to increase the viability of the reaction by ~ 180 mV, which makes it favorable for organic formation.
Once formed, the formate would have a sufficient abiotic chemical potential. For example, it is known that formyl groups form intermediate compounds of the reducing cycle of tricarboxylic acids * and the reducing Acetyl-CoA pathway * , suggesting a possible pathway for the development of biological metabolism.
* — .
- ( - WL-)* — , 2 .Another theory suggests that when heated in the presence of ammonia, which is also a putative component of alkaline waters, formate forms formamide [HC (O) NH 2 ], a highly reactive molecule that is the cornerstone of one of the theories of the origin of life ( Formamide and the origin of life ). The further reaction of this mixture gives hydrogen cyanide (HCN), which is also the basis for another theory of the formation of organic matter ( Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism ). In turn, the dehydration of the formate leads to the formation of carbon monoxide (CO) ( Activated Acetic Acid by Carbon Fixation on (Fe, Ni) S Under Primordial Conditions). As you can see, there are many theories of the origin of organic compounds, and each of them has quite logical explanations.
Although there were several sources of reduced carbon on the early Earth and many possible environments for rich chemicals, the alkaline hydrothermal scenario described above is far more interesting to scientists because of its similarity to the WL-pathway of carbon fixation.
An additional argument in favor of the potential significance of the alkaline hydrothermal scenario for the formation of organic matter is the fact that the WL process is the only one of the six known biological pathways for carbon fixation that releases energy in general, rather than consumes it, and its variations are present in existing representatives of both archaea ( methanogens *) and bacteria ( acetogens * ).
Methanogens * are archaea that form methane as a byproduct of metabolism in anoxic conditions.
Acetogens * are bacteria that release acetate (CH 3 COO - ) as the end product of anaerobic respiration or homoacetate fermentation.The first step in this direction is the reduction of CO 2 with H 2 to form formate (HCOO - or its dehydrated electronic equivalent, ie CO).
This reaction is endergonic * , so some members of both archaea and bacteria use either electron bifurcation * or chemiosmosis * across the cell membrane to trigger this process.
Endergonic reactions * - chemical reactions that require energy from the outside for their course.
Electron bifurcation * - the mechanism of separation of electrons in a redox reaction.
Chemiosmos * - transformation of the energy of the electron transport chain into the energy of ATP (adenosine triphosphate).However, in the absence of cellular fusion mechanisms such as electron bifurcation or chemiosmosis, this first endergonic stage is a key energy bottleneck in the WL pathway and remains a major open question in studies of the origin of biological carbon fixation.
In this study, researchers demonstrated abiotic indirect reduction CO 2 to HCOO - using H 2 induced pH gradient in the microfluidic sediments Fe (Ni) S, through a mechanism that resembles a flow path separated WL electrons.
Research results
First of all, a laboratory equivalent of an alkaline hydrothermal medium was prepared with a simulation of the interface between the Earth's crust and ocean waters. The alkaline component included Na 2 S (100 mM), K 2 HPO 4 (10 mM) and Na 2 Si 3 O 7 (10 mM) in deaerated water. The ocean analog included FeCl 2 (50 mM) and NiCl 2 (5 mM). Both fluids were fed to a Y-shaped borosilicate microfluidic reactor ( 1B ).
Ambient pressure of H 2 and CO 2 was insufficient to reduce CO 2 emissions, therefore, instead of trying to dissolve any gas by bubbling * before the reaction, it was decided to use microfluidic pumps operating on gas pressure.
Bubbling (bubbling) * - the process of passing gas through a layer of liquid.The alkaline liquid was pushed out by H 2 at a pressure of 1.5 bar, and the ocean analog was pushed out by CO 2 at the same pressure.
Each cycle of the reactor was divided into two successive stages: first, for the deposition of Fe (Ni) S deposits at the junction (at the interface) of two liquids; the second ("post-precipitation") - to try to create a reaction between CO 2 and H 2 (or other reagents).
As a result of the interaction of an alkaline liquid and an oceanic analog for 15-60 seconds at the stage of precipitation, a sediment with a width of 30 to 60 μm was formed at the interface between two liquids, visible under a digital optical microscope (in the center at 1B). Removal of metals from the ocean analogue side after precipitation prevented an increase in sediment to the critical value of the reactor channel shut-off.
After the formation of sediment and to prevent clogging of the microfluidic channels by further sedimentation in the second stage, the oceanic fluid was switched to clean deaerated water displaced by CO 2 (on the right in 1B ). In this case, the analog of the alkaline liquid remained the same with Na 2 S, K 2 HPO 4 and Na 2 Si 3 O 7 pushed out by H 2 .
Next, the pH level of the incoming liquids at the entry point was determined: the analogue of the ocean - pH 3.9, alkaline waters - pH 12.3. At a flow rate of 5 μL / min for each inlet, the residence time of liquids in the central channel was ~ 3.3 s, so the system was allowed to run for at least 2 minutes before collecting the output data. Next, the total reactor output (liquid mixture) was collected and analyzed by NMR spectroscopy. The analysis showed that the average value of the concentration of HCOO - was 1.5 μM.
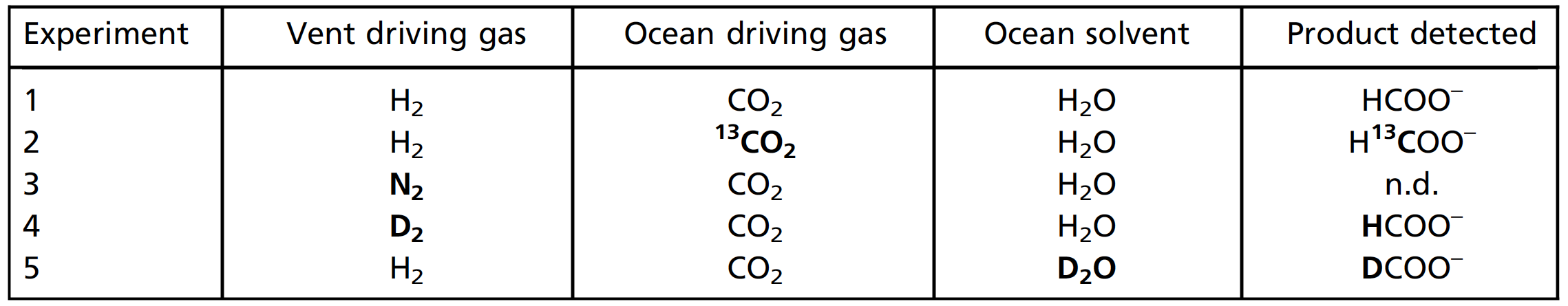
Table 1: experimental results.
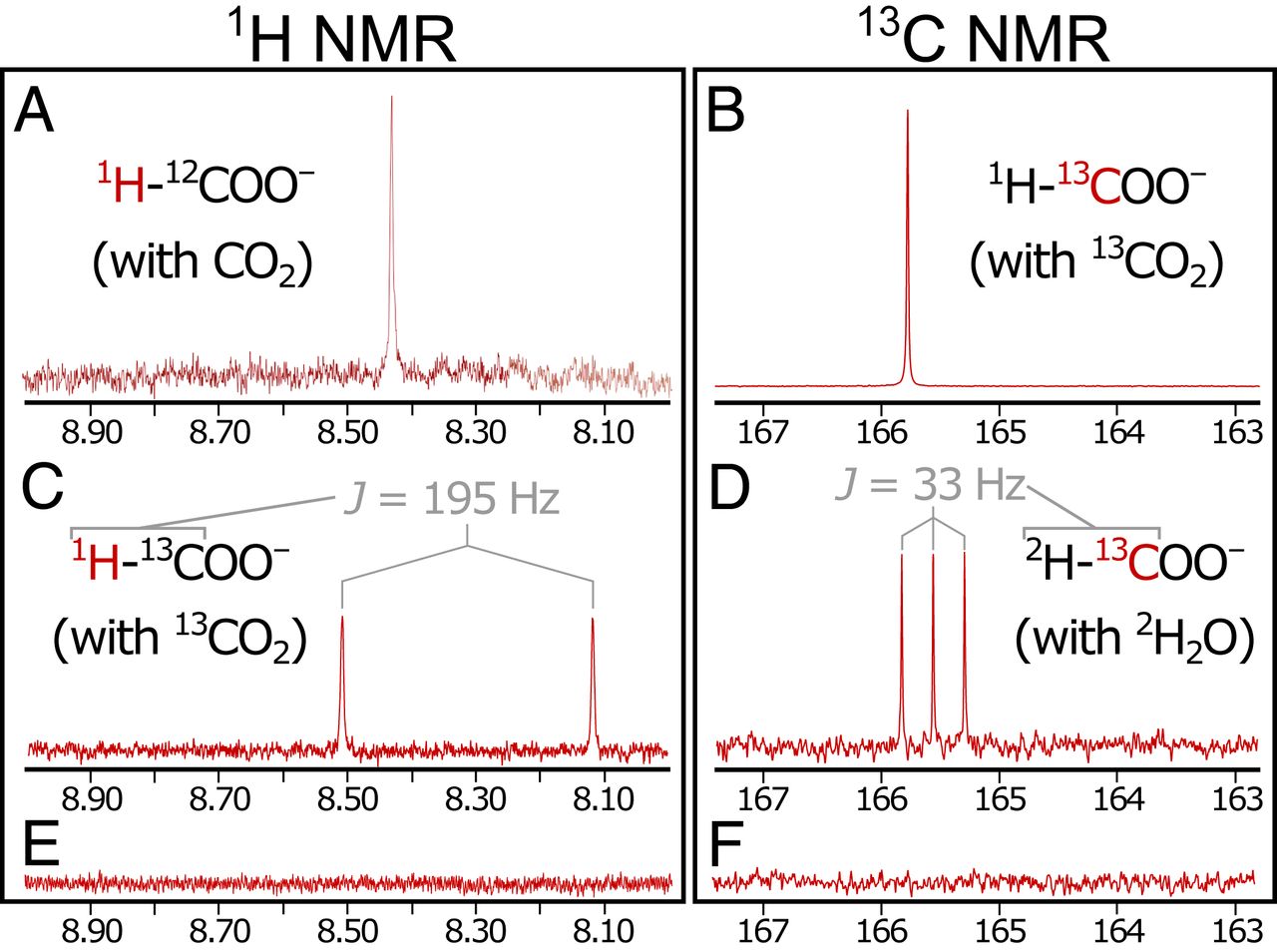
Image # 2
Singlet peaks in 1 H NMR spectra (8.42 ppm (parts per million); 2A ) and 13C NMR (165.8 ppm) corresponds to pure (> 98%) formic acid samples. Performing the steps of precipitation and reaction with isotopically enriched (99% 13 C) 13CO 2 (experiment 2) gave a stronger singlet in the 13 C spectrum (165.8 ppm; 2B ) and the expected splitting of the formyl singlet into a doublet (a signal split into two peaks) in spectrum 1 H (J = 195 Hz) due to the interaction of 1 H - 13 C in the formyl group ( 2C ).
As it turns out, H 2 is needed to reduce CO 2 emissions . With liquid at the outlet side controlled by N 2 instead of H 2(ie, in the absence of H 2 both during and after precipitation), no reduction products were detected (experiment 3; 2E and 2F ).
For a more detailed understanding of the ongoing process, additional experiments on labeling with deuterium ( 2 H or D) (experiments 4 and 5) were performed using isotopic variants throughout all experiments.
Regardless of whether the used untagged H 2 (Experiment 1) or D 2 (experiment 4) for controlling the pump on the side of the alkaline liquid only observed nonisotopic marked HCOO - a fluid outlet. This observation suggests that the reduction in CO2 can occur exclusively on the ocean side.
Conversely, with D 2 O used in place of conventional H 2 O on the ocean side and with unlabeled H 2 driving a pump on the alkaline liquid side (Experiment 5), exclusively deuterated formate (DCOO - ) was found, as evidenced by triplet at 13 C NMR (J = 33 Hz) and no other noticeable peaks ( 2D ). This further confirms that the CO 2 reduction corresponds to the isotopic composition on the ocean side and not on the crustal side.
At the next stage of the study, the role of the pH gradient of the simulated underwater alkaline hydrothermal system was tested. The successful CO 2 reductions shown in Table 1 occurred at an ocean analog pH 3.9 and a discharge analog pH 12.3.
On mixing, this initial ∆pH of 8.4 units would inevitably decrease, but pH gradients of several units are successfully maintained over time on microfluidic scales, especially in the presence of precipitate at the interface.
It was necessary to understand whether such a pH gradient in the reduction system is required to facilitate the oxidation of H 2 on the alkaline side and reduction of CO 2 on the acid side ( 1A). After sedimentation under the same conditions as for experiment 1, the effects of different pH levels and composition of each of the two liquids were evaluated (table # 2). Replacing the simulant alkaline source with pure H 2 O driven by H 2 was ineffective (Table 2, Experiment 6).
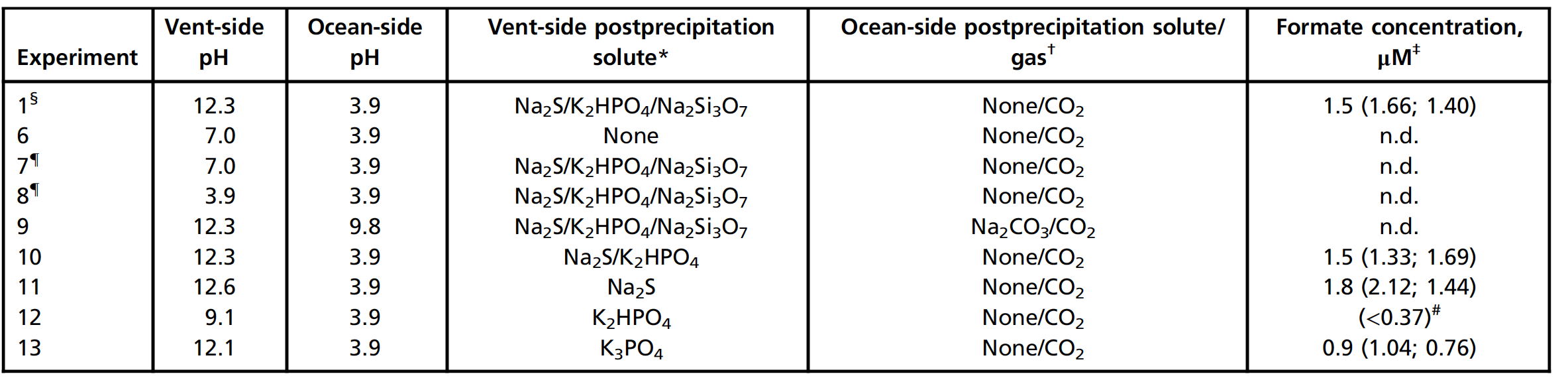
Table 2: results of experiments with different pH values.
Likewise, acidification of the alkaline source analogue liquid with HCl to pH 3.9 and pH 7.0 did not lead to the formation of formate (experiments 7 and 8).
Adding 100 mM Na 2 CO 3 to Oceanic Fluid While Using CO 2as a propelling gas (Experiment 9) raised the pH of the ocean to 9.8 and no product was found under these conditions. Removal of silicate from the source side after precipitation still gave formate (Experiment 10), as did removing silicate and phosphate with only Na 2 S (Experiment 11).
With only K 2 HPO 4 after deposition on the alkaline source side, only residual amounts of formate (below the quantification limit of 0.37 μM) were detected, possibly due to insufficient alkaline pH 9.1 (Experiment 12). The more alkaline K 3 PO 4, on the other hand, raised the pH to 12.1 and led to the formation of significantly more formate (experiment 13).
The scientists say they cannot completely rule out the possibility that precipitate-bound sulfide acts as a reducing agent in addition to H 2 . However, the above results simultaneously confirm the role of the pH gradient and show that continuous feed of aqueous sulfide is not required in the system.
Removal of Ni from ocean sedimentary fluid (Experiment 14) resulted in only a small amount of formate being formed. Conversely, replacing Fe to leave Ni as the only metal in the ocean sedimentary fluid (NiCl 2 , 55 mM; experiment 15) yielded 1.4 μM formate, indicating a decisive role for Ni in sediment composition.
Removal of FeCl 2 and NiCl 2from oceanic fluid, as expected, did not lead to the formation of detectable formate and sediment (Experiment 16).
Scientists believe that the most appropriate explanation for what is happening is the electrochemical process ( 1A ), but there are several alternative mechanisms for reducing CO 2 emissions associated with the oxidation of H 2 , which are less likely.
One of these mechanisms can be called the "simplest", but also the least biochemically homologous - carbon reduction due to direct hydrogenation ( 3A - 3C ). In this case, hydrogen from H 2 will be transferred directly to CO 2either as atomic hydrogen (classical hydrogenation) or as a hydride (ionic hydrogenation).
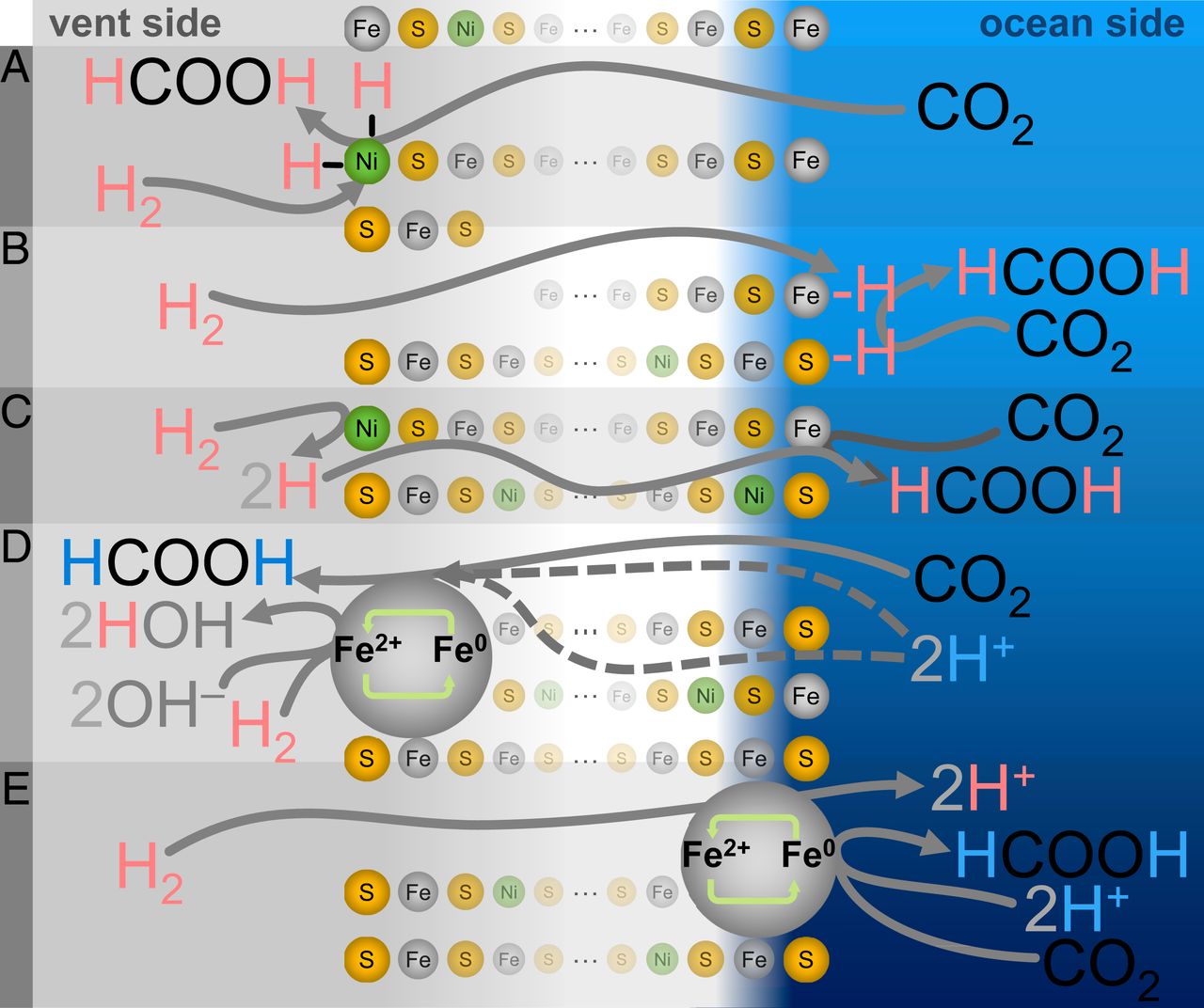
Image # 3
In other words, the output from such a mechanism must correspond to the isotopic signature of the H 2 / D 2 gas released . Instead, the formate produced in this case matches only the isotopic composition of the water on the ocean side, regardless of the composition of the gas or water on the hydrothermal source side.
In direct hydrogenation mechanisms, adsorbed hydrogen particles can exchange with the surrounding liquid so that the original isotopic signature is lost. However, any such process involves the migration of a significant amount of liquid through the sediment. Substantial mixing of the liquids should have caused a mixed H / D signal of formyl, which was not observed in practical experiments, completely excluding hydrogenation.
Another possible option is that the hydrogen atoms in the resulting formate may not come directly from H 2 . Instead, the mechanism can be carried out through the redox cycle of the edge or corner Fe or Ni atom (M 2+ ⇄ M 0 ), in which the metal is first reduced by H2 (leaving two protons for dilution), and then the metal transfers the acquired electrons to CO 2 with the accompanying removal of protons from the local aqueous medium ( 3C - 3E ).
However, this scenario is difficult to compare with the real pH values that were during the experiments. The pH of 3.9 was achieved solely by dissolving CO 2 in water. Thus, any protons from the ocean side should occur as a result of the dissociation of carbonic acid through:
H 2 O + CO 2 ⇌ H 2 CO 3 ⇌ H + + HCO - 3
When the reaction was carried out using D 2 O (Experiment 5) as the solvent on the ocean side, only DCOO - was found in the output stream . It follows that the CO 2 reduction did not occur on the source side where normal water (H 2 O) and H 2 were present .
There may be several scenarios for such a localized redox cycle ( 3D and 3E ), but since they all require joint placement, none of them can offer an isotopic signature exclusively on the ocean side, which was observed during the experiments.
Combined with the strong pH dependence of the reaction, these results suggest that CO 2 reduction occurs via an electrochemical mechanism, in which electrons from the oxidation of H 2 on the alkaline source side move through the Fe (Ni) S precipitation towards CO 2 on the ocean side. ( 1A ).
The above processes could not have occurred if there was no mechanism to activate and maintain the interaction between ocean water and alkaline hydrothermal vents. In addition, the question arises regarding the "vitality" of the formed organic compounds, since they could simply dissolve in the ocean water before they take on any biochemical role.
The answer to the first question may be the Venturi effect * caused by the increased porosity of the structure of hydrothermal vents. Once inside the vent, ocean carbon dioxide can react with electrons transported through the catalytic minerals of the hydrothermal vent channel, and new sediments can also occur further in time when the two liquids come into contact.
The Venturi effect * is the pressure drop when a liquid or gas stream flows through a constricted part of a pipe.Modeling of this theory showed that in the case of an experimental reactor 300 μm wide, microfluidic fusion of two reagents actually occurs, which was shown in the course of practical experiments.
Scientists also note that this effect is not limited to underwater alkaline vents and is likely to occur in porous hydrothermal systems anywhere and at any depth, allowing for a variety of geochemical scenarios for the origin of life.
It should be noted that the microfluidic system for the reduction of CO 2 using H 2 is not the only one. There is also a technique that uses a single-channel periodic system.
Other minerals (Fe 3 Ni), higher pressure (10 bar for H2 ) and higher temperatures (100 ° C) than in the experiments carried out, the batch system allows to obtain much more formate, as well as several products of further reduction (acetate, methanol, and pyruvate). Moreover, the rate of formate production (5.21 x 10 -9 mol / s) is four orders of magnitude higher than the rate achieved by the microfluidic system.
The importance of the periodic system lies in the fact that its results confirm the performance of the microfluidic system. This confirms the theory regarding the existence of organic matter in the conditions of anoxic alkaline hydrothermal vents.
For a more detailed acquaintance with the nuances of the study, I recommend looking at the report of scientists andadditional materials to it.
Epilogue
Everything that has a beginning has an end. These words, spoken by the Pythia from the film "Matrix", in one interpretation or another, were spoken by real philosophers and scientists long before the release of this motion picture. In addition, one of the fundamental principles of science is recalled from the course of school chemistry - nothing disappears anywhere and is not taken from anywhere without a trace. With what will happen at the end, or rather after it, humanity will have to deal with it for a very long time. But there is already some understanding of what was in the beginning.
In this study, scientists described a possible variant of the formation of the first organic compounds. In their opinion, this process took place at the junction of ocean waters and hydrothermal springs. During the experiments, it was possible to convert CO 2 into organic molecules by means of H 2and controlled pH.
This result not only explains the origins of life on our planet, but also can be used in the development of instruments for reducing emissions of CO 2 , which is a very distressing problem of the modern world. Among other things, understanding how organics appeared on Earth allows us to build more cost-effective theories about the possible presence of it on other planets like ours.
If we switch to a more philosophical wave, then we can say with confidence that this work demonstrates the importance of understanding the past for the successful formation of the future. History is replete with research, which contemporaries called idle curiosity and a waste of time. The vast majority of them turned out to be much more important than anyone would have guessed. The conclusion is simple: in science, you need to look for answers to all questions, no matter how stupid they may seem at first glance.
Thanks for your attention, stay curious and have a good work week, guys. :)
A bit of advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting content? Support us by placing an order or recommending to friends, cloud VPS for developers from $ 4.99 , a unique analogue of entry-level servers that we have invented for you: The Whole Truth About VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server correctly? (options available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Is Dell R730xd 2x cheaper in Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - From $ 99! Read about How to build the infrastructure of bldg. class with the use of Dell R730xd E5-2650 v4 servers at a cost of 9000 euros for a penny?