
As a person with non-standard dimensions (a la mop with ears), I know perfectly well how difficult it is sometimes to choose any item of the wardrobe of the right size. Apparently, some of the scientists at Harvard University also faced a similar everyday problem, because in their recent research they describe a new type of material with shape memory. The basis of this innovation is a protein that is easily found in hair, nails and skin - keratin. How exactly was the favorite of marketers of cosmetics manufacturers used, what kind of metamorphosis is the new material capable of, and what are the options for using the metamorphic material? To get answers to these polls let's dive into the report of scientists. Go.
Research basis
Over the past few years, interest in materials capable of remembering certain shapes given during production has increased significantly. Such materials can be used in medicine, construction and aerospace industries, etc. However, as scientists themselves declare, the degree of interest in such developments cannot be equated with the degree of information available about them. In other words, such materials still have many unsolved secrets.
Metamorphic materials are most often associated with synthetic substances; however, natural structures also have similar properties, which is due to the structural metastability of the secondary structures of proteins. For example, keratin α-helices, arranged in a helical coil, are known to undergo continuous structural transition to metastable β-sheets when a load is applied along their longitudinal axis. Depending on the type of α-keratin, this process can be irreversible or reversible, and in the second case it resembles the martensitic * shape memory mechanism of metal alloys.
Martensitic transformation * is a polymorphic transformation in which a change in the mutual arrangement of atoms (or molecules) of a crystal occurs by their ordered movement.In biological materials (animal skin, for example) such a transformation mechanism is due to the need for protection and physiological functioning in response to an external stimulus.
In this work, scientists tried to implement the reversible transition of keratin from the α-helix to the β-sheet. In their opinion, it is this process that is the main mechanism for creating a high-tech nanostructured material with shape memory, which uses hydration as a trigger and is by its nature biocompatible and biodegradable.
Research results
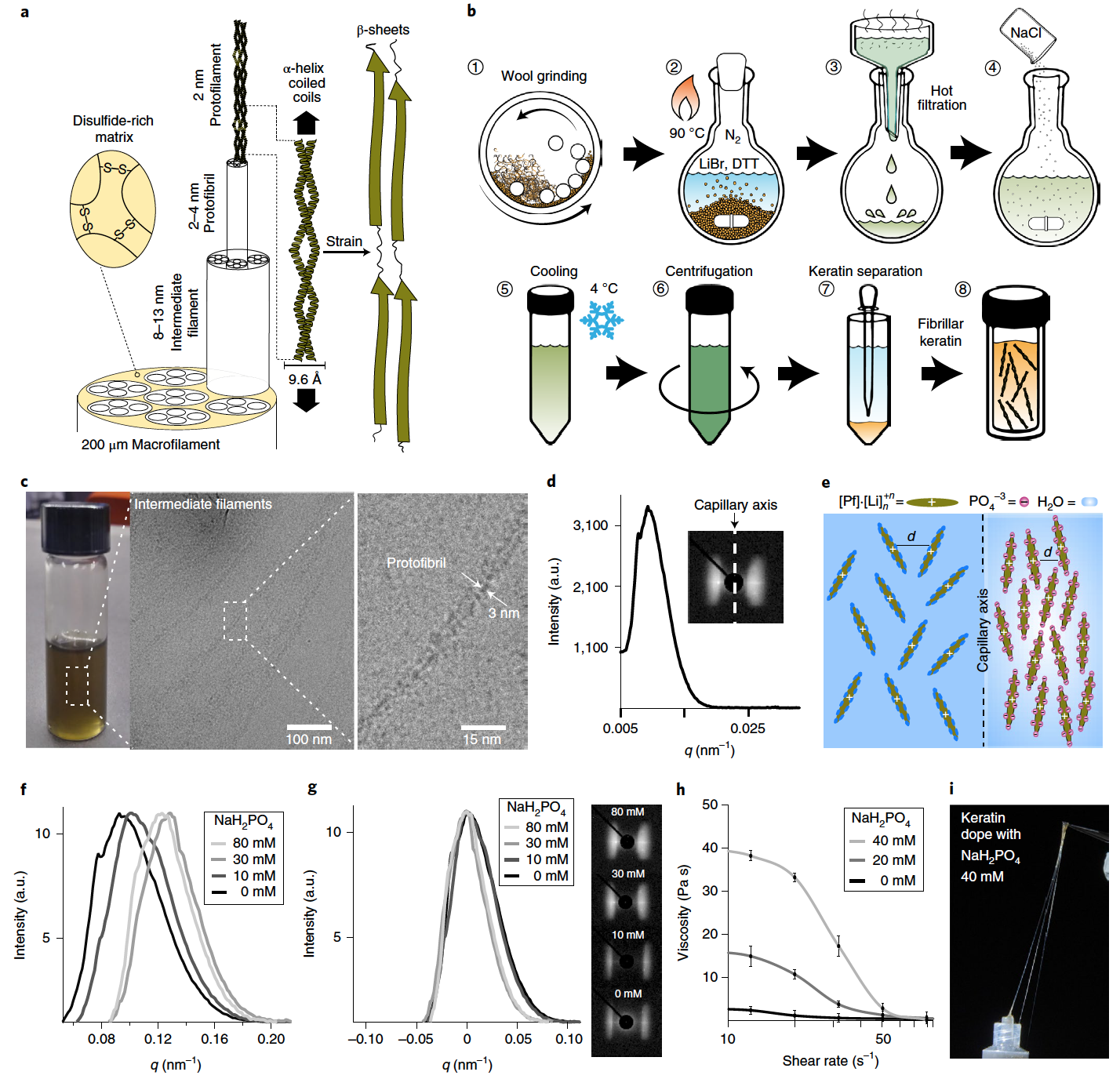
Image No. 1
In animal hair, the deformation-induced transition from the α-helix to the β-sheet is possible due to the paired configuration of α-helices in the architecture of the spiral coil * ( 1a ).
Spiral coil * ( Coiled coil ) - structural motif proteins, when 2 to 7 alpha helices intertwine both strands of the rope.The spiral coils self-organize hierarchically into an anisotropic fibrillar structure that ranges from protofibrils * to macrofibrils * , which ensures the continuity of mechanical transformation at all spatial scales.
Protofibrils * are the thinnest protein filaments that make up the bulk of myofibrils.In this study, fibrillar keratin was extracted from angora wool using lithium bromide (LiBr), a salt that can induce a reversible solid to liquid phase transition of crystalline keratin in water.
Myofibrils * - contractile filaments in the protoplasm of striated muscle fibers of skeletal muscles, heart muscle and muscles with double oblique striation.
Another requirement for the release of fibrous keratin from the hair structure is the disruption of the dense disulfide network of the hair matrix component. This was achieved using 1.4-dithiothreitol (DTT, C 4 H 10 O 2 S 2 ), which is capable of cleaving the disulfide bond to form two sulfhydryl moieties. This reaction is reversible under oxidation conditions, which allows natural disulfide bridges to be restored during fabrication.
The keratin was then successfully recovered by treating the wool with an aqueous solution of LiBr and DTT at high temperature ( 1b ). At room temperature, the keratin was finally isolated by liquid separation, resulting in a highly concentrated keratin solution with a shelf life of several weeks in the absence of oxygen.
Raman spectroscopy and circular dichroism were also performed, which confirmed the presence of helical coils of α-helices. Evidence for a hierarchical structure of keratin down to the protofibrillar level was confirmed by cryogenic transmission electron microscopy (cryo-TEM). During microscopy, it was found that the length of the nodes is within a few micrometers, and the width is about 10 nm ( 1c), which fully corresponds to the structural features of the intermediate fibers. It was also possible to establish that the hierarchical structure of intermediate filaments consists of packed protofibrils with a constant width of ~ 3 nm.
The implementation of a hierarchical keratin architecture that can provide long-range ordering of the α-helix executive units requires the imposition of anisotropic alignment of protofibrils during the manufacturing process. It has been found that keratin protofibrils self-assemble into a nematic crystalline phase under the influence of shear stress and spatial constraints. This fact was established by observing the anisotropic nature of synchrotron X-ray scattering, which was obtained from a sample of keratin solution (401.7 mg / ml) prepared in a quartz capillary ( 1d).
During the analysis, the capillary was positioned perpendicular to the X-ray beam, and its longitudinal axis was parallel to the meridional axis of the detector. The equatorial nature of the scattering suggests that the keratin domains were predominantly oriented parallel to the capillary axis (inset at 1d ). The average distance between keratin domains is related to the lattice size parameter ( d ), which is obtained from the maximum intensity of the scattering vector modulus ( q ): d = 2 π / q .
Scientists suggest that the nematic ordering of the keratin protofilament * is the result of the shear stress that is created on the capillary wall during sample preparation and is also the result of further stabilization due to the limited space.
Protofilaments * are filamentous protein structures that are building blocks of microtubules (protein intracellular structures that make up the cell frame).In such a case, the increased rigidity and self-assembly of keratin protofibrils is expected to result in a higher degree of ordering in the nematic (filamentous) phase. Control over the self-organization of the keratin liquid crystal phase was achieved by stimulating protein-protein interactions through a charge screening effect. Due to the presence of lithium cations, which are absorbed on the surface of the protein, keratin will have a net positive charge. And the phosphate anion was used because it has a high screening effect with respect to positively charged surfaces ( 1e ).
The addition of sodium dihydrogen phosphate (NaH 2 PO 4) caused the packing of the nematic phase of keratin to tighten, which is indicated by the shift of the peak towards a higher q ( 1f ) value .
The addition of kosmotropic * salt also resulted in a narrowing of the equatorial scattering pattern and, as a consequence, an exacerbation of the scattering peak, which indicates an increase in the alignment of the keratin domain along the capillary axis ( 1g ).
Cosmotropic * are co-solvents if they contribute to the stability and structure of water-water interactions. Cosmotropes cause an increase in the interaction of water molecules, which also stabilizes intramolecular interactions in macromolecules such as proteins.With an increase in the concentration of NaH 2 PO 4, the aggregation of protofibrils causes an increase in the viscosity of the protein solution at low shear rates ( 1h ). However, with increasing shear rate, the alignment of keratin protofibrils causes a sudden decrease in viscosity, which gives the protein solution a pronounced pseudoplasticity * .
Pseudoplasticity * - occurs when the viscosity of a fluid decreases with increasing shear stress.At a NaH 2 PO 4 concentration of 40 mM and a protofilament concentration of 401.7 mg / ml, the keratin dopant exhibits viscoelastic properties. This is good news for researchers, as fibers can be formed directly by simply pulling on the protein with tweezers ( 1i ). If the concentration of NaH 2 PO 4 is reduced , the keratin solution loses its viscoelastic properties and cannot form fibers directly from the solution.
As noted by the scientists, the alignment of keratin α-helices along the fiber axis is a design criterion that ensures high strength and a high degree of fiber fixation. When the axes of the α-helix are parallel to the tension vector, maximum unwinding of the α-helices can be obtained, which allows the material to increase deformation prior to failure due to plastic deformation and reorganization.

Image No. 2
An aqueous solution of NaH 2 PO 4 was used as an anti-solvent, which made it possible to achieve external diffusion of LiBr from the extruded keratin dopant and further self-assembly of the protein due to the charge screening effect ( 2a). The reduction of the disulfide covalent network became possible due to the oxidative activity of hydrogen peroxide (H 2 O 2 ) towards the thiol group of cysteine. The high concentration of protein in the dope gives the fiber strength during the coagulation process, which enables a flexible and reliable spinning process.
As a result, long and strong fibers ( 2b ) can be obtained , and the high production rate allows fibers with a diameter of 10 μm to be achieved.
The nematic phase organization of keratin protofibrils results in a fibrillation process that generates hierarchically structured and anisotropic fibers. The scanning electron microscope showed that one fiber consists of continuous fibrils, the length of which is at least several tens of micrometers ( 2b ). It was also observed that fibrils with a diameter of 50 nm are the core of the resulting fiber ( 2d ).
Polarizing optical microscopy confirmed the anisotropic nature of the fiber core, which was established by observing birefringence with a maximum transmitted light intensity at an angle of 45 ° ( 2e ).
The structure of the spiral coil has an anisotropic architecture, it was established using wide-angle X-ray scattering (WAXS ). The two-dimensional scattering profile shows a characteristic equatorial reflection at 9.65 Å, which corresponds to the distance between the axes of adjacent α-helices ( 2g ). One-dimensional analysis along the meridian axis shows the presence of characteristic meridional (at 5.15 Å) and extra-meridian (5.05 Å) reflections, which correspond to the projection of the α-helix pitch ( 2h ).
It was also found that the maximum is shifted towards higher q values , i.e. there are unfolded peptide chains oriented parallel to the fiber axis and probably forming a β-layer conformation (2i ).
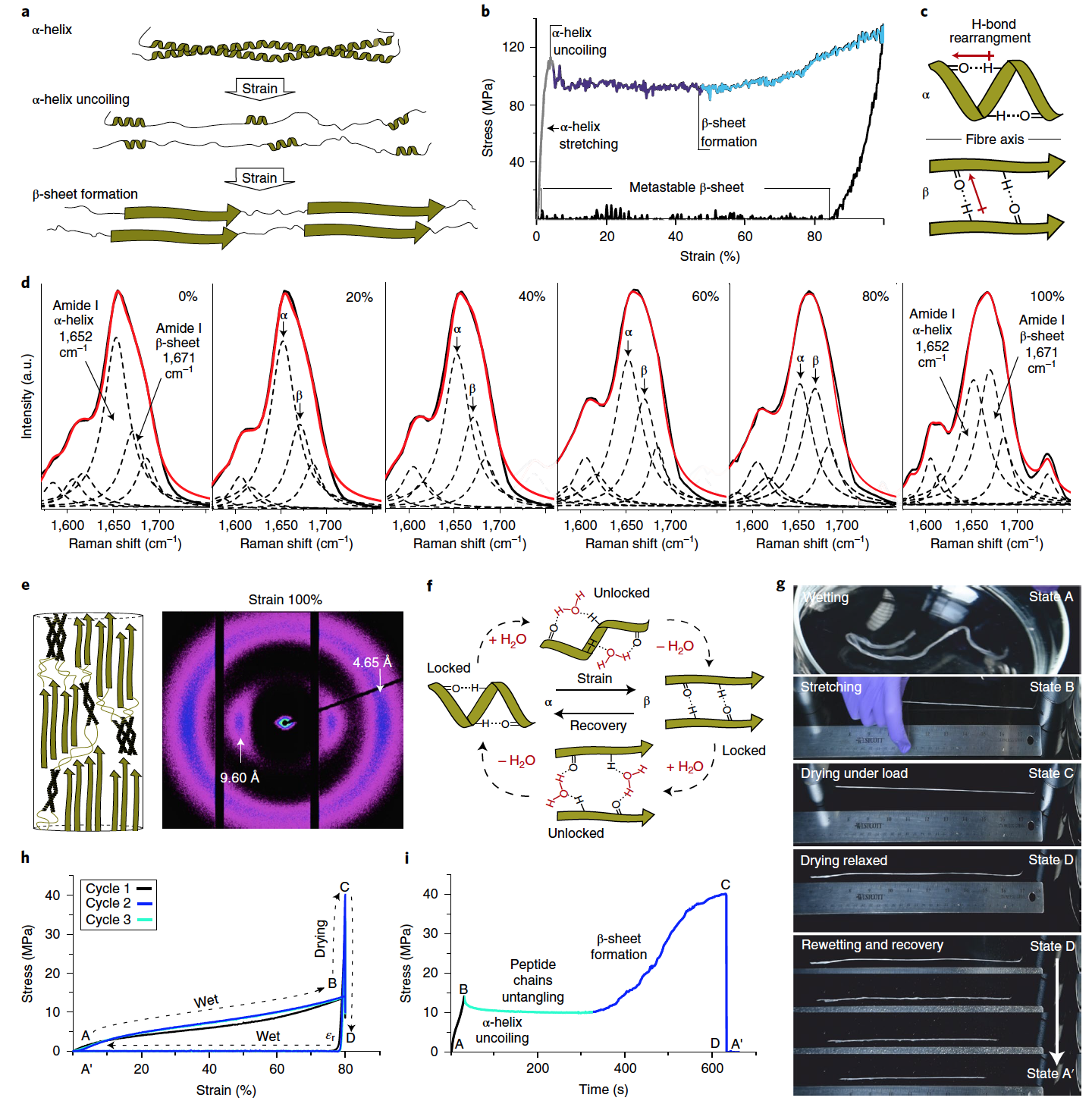
Image №3
At the next stage of the study, we studied the shape memory fibers that respond to hydration.
The shape memory effect of engineered keratin fibers is based on the reversible unwinding of the α-helix and the formation of metastable β-sheets in uniaxial deformation ( 3a ).
Tensile tests carried out on individual keratin fibers have confirmed this mechanism. The initial elastic state was established up to ~ 5% deformation (Young's modulus = 4.18 ± 0.10 GPa). This is followed by a region characterized by a constant yield point (96.1 ± 3.1 MPa) ( 3b ). This reaction corresponds to the process of unfolding the α-helix.
As the stress increases further, the unfolded and extended peptide chains of keratin are stabilized in their extended geometry by assembling into β-layers ( 3c ). This region of β-sheet formation is characterized by strain hardening at a deformation of ~ 50%, since the applied load is not only dissipated due to the destruction of the coiled coils, but is also transferred by stretching the β-sheets. When the load is removed at 100% deformation (tensile strength 137.18 ± 1.03 MPa), the fiber exhibits plastic deformation (~ 85%), which is consistent with the transformation of unfolded keratin chains into new metastable β-sheets. The mechanical properties of the obtained keratin fibers fully correspond to those of natural wool.
It is important to note that the unstressed fibers also contain an insignificant proportion of β-sheets ( 3d ); however, at 100% deformation, a significant increase in the β-sheet component is observed. The WAXS analysis confirmed the presence of deformation-induced transition from the α-helix to the β-layer ( 3e ).
In stretched fiber, β-sheets are kinetically stable due to the presence of a network of hydrogen bonds, which prevents them from converting back to more thermodynamically stable α-helices. It is this property that makes it possible to create a system with a shape memory cycle, in which the network of hydrogen bonds plays the role of a blocking mechanism to ensure the fixation of the deformed shape.
In the course of the experiments, water played the role of a stimulus contributing to fiber deformation and restoration of its original shape ( 3f ). The technique was tested on a bundle of keratin fibers of the same diameter ( 3g and video below).
Demonstration of shape memory using the example of individual fibers.
First, the fiber bundle was hydrated in deionized water for a few seconds (state A), then manually stretched in air while still wet (state B), and then held under load at room temperature for 10 minutes to allow the fibers to dry ( state C).
After removing the weights, which allowed the fibers to become relaxed, there was no visible or noticeable change in length between the stretched and relaxed forms (state D).
If, on the other hand, water is applied to the resulting fibers (by spraying), then the fibers shrink to their original length within a few seconds (state A ').
The use of water in the ongoing metamorphoses greatly facilitates the process of restructuring the protein structure. This is indicated by a general decrease in tensile stress ( 3h ) and a more gradual transition between fiber states.
When the fiber dries under load, β-sheet formation is indicated by a sharp increase in stress corresponding to an increase in fiber stiffness, which can be measured over time as the fibers become dehydrated and hydrogen bonded ( 3i ).
The resulting material, due to the long-range ordering of its fibrillar structure in the dry state, demonstrates tensile strength (137.18 ± 1.03 MPa) and Young's modulus (4.18 ± 0.10 GPa), which is much better than that of the previously developed prototypes. When hydrated, the tensile strength is 14.94 ± 0.46 MPa, which is also significantly superior to other developed materials.
In addition to properties and characteristics, the developed material has one more advantage over its competitors - the possibility of its use in 3D printing.

Image No. 4
Basic geometric shapes can be obtained by extrusion of the protein dopant into a hydrogel, which serves as a support and coagulation bath ( 4a). The properties of keratin allow the use of small needles, which allows the creation of structures on a scale of approximately 50 μm ( 4b ).
The alignment of keratin protofibrils follows the extrusion path in 3D printing and therefore leads to highly ordered architectures that are characterized by an internal structural hierarchy from molecular to macroscopic level ( 4c ).
After the desired sample has been 3D printed, it is necessary to achieve a constant shape fixation. This requires the formation of disulfide bridges by oxidation induced by H 2 O 2 . Before the oxidation process, the samples can still be manipulated by changing their shape due to their plasticity.
For example, during the tests, a star (origami) was manually made from the printed sheet, which subsequently passed the stage of fixing the shape by oxidation in H 2 O 2 and NaH 2 PO 4 ( 4d ). Therefore, it is not necessary to print the desired shape immediately, it can be done after printing and before the fixing step ( 4e ).
Demonstration of form memory using the example of a printed sample.
Like the fibers previously tested, the printed figurines have the same moisture-sensitive shape memory properties. The star-shaped origami architecture was chosen to demonstrate the effectiveness of the shape memory mechanism when performing fairly complex geometric transformations, according to scientists.
When hydrated, the 3D printed origami model is malleable and can be unwrapped and arbitrarily transformed, for example, into a rolled tube (left at 4f). As it dries, the square sheet loses its plasticity and is fixed in its new temporary shape. The restoration of the star-shaped origami architecture is then triggered by rehydration, which occurs within a few seconds due to the high surface-to-volume ratio causing the keratin to be rapidly exposed to water (right at 4f ). In other words, the printed sheet first unfolds to its previous configuration and then folds itself into a star shape.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists and additional materials to it.
Epilogue
In this work, scientists have demonstrated a new type of material with shape memory, activated by contact with water. The basis of the process of spontaneous transformation of one given form into another is the transition of α-helices of keratin to β-sheets.
The resulting material can be used in 3D printing, while you can initially set the desired shape or do it after printing a regular sheet. Changing the shape after printing is possible due to the plasticity of the obtained sample, the shape of which can be fixed already at the stage of oxidation. This two-step process enables the creation of highly complex shapes with customizable structural features down to the micron level.
The authors of this study say that the range of applications for their development is quite large. Shape memory materials can be used both in light industry (for example, a T-shirt that changes size to your liking) and in medicine (activating fabrics).
The results of the experiments are good enough, but scientists intend to continue to conduct experiments, because materials, the architecture of which can change depending on external influences and at the request of a person, are a very interesting subject for study.
Thanks for your attention, stay curious and have a good work week, guys. :)
A bit of advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting content? Support us by placing an order or recommending to friends, cloud VPS for developers from $ 4.99 , a unique analogue of entry-level servers that we have invented for you: The Whole Truth About VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server correctly? (options available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Is Dell R730xd 2x cheaper in Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - From $ 99! Read about How to build the infrastructure of bldg. class with Dell R730xd E5-2650 v4 servers at a cost of 9000 euros for a penny?