
If you are interested in biology, especially if you are teaching this subject, then my article on the preparation and conduct of laboratory work to identify a genetically foreign gene in the DNA of food will be useful to you.
The controversy surrounding GMOs, as well as the incessant scientific and ethical discussions about the legality of human intervention in the "affairs of Nature / God", will be left to the philosophers. We only state that the professional competence of a modern biologist, biotechnologist, bioinformatics and genetics is inconceivable without the ability to research and, if necessary, change genes. Already at school, and later at university, biotechnological experiments and experiments require pupils and students to set clear tasks, understand the methodology of the experiment, and the ability to analyze data. All this develops curiosity and confidence, creates a foundation for further exploration of issues and problems associated with scientific research. My PLAN-CONSPECTbased on 3 tests carried out with BioRad reagents and standard equipment required for PCR analyzes. But, of course, any other KITs can be used to detect GMOs. All the nuances of the experiment (protocols) are detailed in the documentation accompanying the reagents, so I will not dwell on them in detail. So, the outline plan carries 2 names: PLAN - because it is required to clearly and minutely plan the content of the lesson, and CONSPECT - because it is necessary to think in advance what material to teach the children and what to focus on in order to fully reveal the purpose of the experiment.
Plan
The experience is designed for two sessions.
Lesson one:
1. Theoretical familiarization of students with the basics of GMO detection strategy (targets for detection and identification).
2. Isolation of DNA from food (a schoolchild or student brings any product of his choice).
3. Carrying out the polymerase chain reaction.
Second lesson:
4. Gel electrophoresis and discussion.
5. Concluding remarks from the teacher.
Abstract
First lesson
1. Where do new genes and auxiliary elements come from (15-20 minutes)
Genetically modified organisms are organisms that have an alien combination of genetic material obtained using modern biotechnology. For example, using Ti plasmids, foreign genes and characteristics of genetically modified plants can be genes that provide resistance to herbicides (cp4, epsps, gox), resistance to pests (cry), or genes that change product quality (PG, Bay TE). For the expression of a foreign gene in a host cell, additional genetic elements are required:
- promoter - a nucleotide sequence that is recognized by RNA polymerase as a marker of transcription initiation;
- – , - ;
- - – ( ), ;
- – , , .
The plasmid construct also contains a highly conserved chloroplast gene from Photosystem II - part of the light reaction of photosynthesis to confirm that viable DNA has been extracted and that a negative GM result is not associated with a non-viable matrix.
The identification of recombinant DNA in food can be done in several ways, as shown in Figure 1.

Figure 1. Identification of recombinant DNA in food
The most common ready-to-use reagent kits used to identify GMO products are based on the identification of a promoter and terminator. BioRad's GMO Investigator Kit uses PCR and DNA electrophoresis to check for the presence of two different DNA sequences associated with GMOs: the 35S promoter from the cauliflower mosaic virus and the nopaline synthase gene terminator from Agrobacterium tumefaciens. These DNA sequences are found in> 85% of genetically modified plants marketed worldwide. The new gene present in the DNA of the positive control is epsps. As a control of the integrity of plant DNA extracted from food, PCR is used to amplify a portion of the photosystem II chloroplast gene that is common to most higher plants. So, the test targets 3 targets: a promoter,terminator and part of the chloroplast gene of photosystem II, respectively, in a set of 3 primers, two of which (primers of the promoter and terminator) were mixed. Therefore, there are 2 vials in the set: red - GMO primers, used to determine if the food contains GMOs, and green - plant Photosystem II, used to determine if DNA has been extracted from plant material.
After the introductory speech, students should be familiarized with the protocol for DNA extraction and PCR, step by step explain the course of the experiment and focus on the features of working with a KIT from a particular manufacturer.
2. Isolation of DNA from food (15-20 minutes)
Depending on the available jobs, children can be divided into groups of 2-3 people, each of whom chooses an interesting food product for analysis. Chips or popcorn, so beloved by schoolchildren, are welcome as research material, since almost all potatoes and corn from which crispy goodies are made carry foreign genes and it is very easy to identify GMOs in them. Or berries, for example, strawberries, blueberries, bread with sunflower seeds. But it is advisable to agree in advance who will test which product, because KITs for DNA extraction are designed for certain products and, for example, the one used in this case does not imply the isolation of DNA from flour, oil, seasonings, corn flakes, etc.
In the experiments I have described, DNA was isolated from blueberries, cucumbers and sunflower seeds.
Distinctive features of this study:
- Pre-weighing of the sample (1 g) and thorough grinding with a mortar. Grinding is necessary to destroy the rather dense cell walls of plants (which animal cells do not have).
- Isolation with a homogenized InstaGene Matrix (although of course a detergent can be used) without the addition of proteases (destruction of cellular proteins) and without precipitation of DNA with salts, since the salt is already in InstaGene.
- There is no sorbent in KIT, therefore, without elution - in principle, children can not pay attention to this.
Why was InstaGene Matrix used in principle? Because this is a fairly fast way to isolate DNA - within 15-20 minutes, which significantly saves class time. InstaGene also chelates divalent ions (eg Mg2), which are required for enzymes that destroy DNA (eg DNase).
3. Carrying out the polymerase chain reaction
After the successful isolation of DNA from food by children, according to the manufacturer's protocols, PCR mixtures are prepared for 6 Eppendorf tubes according to Table 1 (10-15 minutes).

Link
After that, the 1st part of the experiment ends. The teacher places the tubes in the amplifier himself and sets the amplification modes according to the protocol.
Second lesson
4. Gel electrophoresis and discussion (40-45 minutes)
Children prepare a 3% agarose gel in TAE buffer. While electrophoresis lasts (200 V 20 minutes), you can discuss the likely options for the results (Figure 2). And after receiving the photos, discuss possible mistakes.

Figure 2. Possible test results
Reference
For example, in the group of children who tested cucumbers, no amplicons were observed (Figure 3).

Figure 3. Gel-image of sample electrophoresis (cucumber)
⁕ - controls (- not containing GMO, + containing GMO); T - test sample; M - molecular weight marker; PMM - photosystem II primer; GMO is a primer for GMOs.
Discussion on the results of 1 sample:in this case, no primers were added to the amplification mixture.
The second experience is testing the DNA of sunflower seeds (Figure 4).
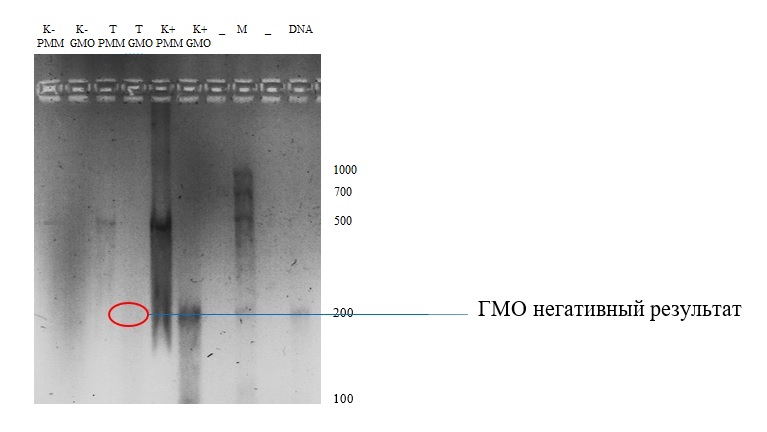
Figure 4. Gel-image of electrophoresis of a sample of sunflower seeds
⁕ - controls (- does not contain GMO, + contains GMO); T - test sample; M - molecular weight marker; PMM - photosystem II primer; GMO is a primer for GMOs.
Discussion on the results of the 2nd sample:the negative control (gel pocket 2), in whose genome there were no modified genes, does not contain amplicons. This indicates that the primer did not recognize the sequencing region of the 35S promoter and the NOS terminator. The opposite pattern with the detection of an amplicon of about 200 base pairs was observed in the 6th gel pocket (positive control - genetically modified control). Visually, only one amplification product with a length of about 200 bp was obtained in the positive control GMO (gel pocket 6), but the amplification products of the promoter and terminator were almost the same size (203 bp and 225 bp, respectively (BioRad)), so that we can assume that there are two amplification products in the gel pocket 6.In most studies, the 35S promoter and the NOS terminator are the most commonly used and can be used to detect modified genes in more than 85% of cases. This method is sufficient to answer the question whether the above promoter and / or terminator was present, but this method is not enough to answer which genes were inserted.
Amplicons specific to the photosystem II chloroplast gene can be found in all 3 food samples (pockets 1, 3, 5), both those containing modified genes and those that do not contain modified genes. The studied samples did not contain the amplicons of the NOS terminator or 35S promoter (pocket 4). Despite the fact that the experiment was carried out successfully and the students received an unambiguous result, the photo is not quite clear, as if it is cloudy. Since this phenomenon extended to the entire gel, it can be concluded that contamination occurred during the preparation of 1x TAE buffer. It was probably contaminated glassware in the laboratory.
The latest experience is testing blueberries (Figure 5).

Figure 5. Gel-image electrophoresis of blueberry sample
⁕ - controls (- does not contain GMO, + contains GMO); T - test sample; M - molecular weight marker; PMM - photosystem II primer; GMO is a primer for GMOs.
Discussion on the results of 3 samples: after running the gel, the agarose gel is viewed from top to bottom. All 3 food samples contain amplicons characteristic of the photosystem II chloroplast gene. A band can be seen in the negative control (with GMO primers), since this is a genetically free control, this is very strange. No amplicons of about 200 base pairs were expected. Band of 200 bp also appears in the test sample (blueberry) and in the positive control. This indicates that the primer recognized the sequencing site of the 35S promoter of the NOS terminator.
But why the test of a sample of blueberries turned out to be positive (genetically modified), this may be due to the fact that blueberries are a natural transgenic plant species.
The sample under study is probably an example of the interference of one organism with another organism using soil bacteria (tumefaciens). One such example of natural transgenic transfer in blueberries has already been identified by Tatyana Matveeva, Doctor of Biological Sciences, Professor at the Institute of Genetics and Biotechnology, Faculty of Biology, St. Petersburg State University. She and her colleagues at the institute compiled a worldwide catalog of plants with genomes already sequenced. Of the 275 plant species studied, 23 were natural transgenes. Includes peanut cultivar, walnut cultivar, hops, tropical guava fruits, clove flowers, Surinamese cherries, cranberries and blueberries. (Matveeva, 2019).
Therefore, there is an assumption that the studied blueberry is a natural transgene.
5. 2
It seems that performing PCR is simple, but troubleshooting can be more difficult if the desired amplicon is not obtained or if non-specific fragments arise. Most often we are talking about contaminated glassware in the laboratory. To avoid contamination of reagents and PCR approaches, care must be taken to use fresh pipette tips for each pipetting process. In addition, it makes sense to change gloves more often as you work. In addition, new and sterilized reaction vessels and solutions should always be used and properly labeled to clearly track contamination. There can be many reasons why PCR does not work. Various chemical and physical parameters must be observed for a successful PCR. Unfortunately, it very often happens that after PCR it is not possible to obtain the desired results.
Since even the smallest amounts of DNA can be detected by PCR, it is extremely important to avoid contamination of PCR reaction mixtures with PCR products from previous experiments or "foreign DNA" from other sources.
Outcome
The experience in detecting GMOs in food is intended to gain practical skills in carrying out the polymerase chain reaction. Despite the fact that a synopsis plan is proposed, designed for 2 lessons, the break between which is at least one day, however, the typical scenario remains at the discretion of the teacher. The analytical sections of this study are designed to guide learners through the process of discovering and understanding concepts that are relevant to procedures and data analysis at every stage of the journey. It is hoped that this approach (compared to the teacher giving the students all the background information) will make the whole study more understandable for a larger number of students. As long as the teacher has the opportunity to check the progress and level of understanding of each group (during 2 lessons),some degree of independence is possible if desired. This approach allows more learners to acquire the desired skills as defined above.
List of used literature:
- bio-rad, „www.bio-rad.com,“ 03 Februar 2020. [Online]
- Matveeva T., Ottem L. (2019). Widespread occurrence of natural genetic transformation of plants by Agrobacterium. Plant Molecular Biology, 101, 415-437. DOI: 10.1007 / s11103-019-00913-y