
Have you ever tried to explain to a three-year-old what atoms are? No? And rightly so, because later the child will run around the house, playground and store, poke a finger on any object and ask, "And here are the atoms?" But seriously, the curiosity inherent in children is what often becomes the driving force behind many discoveries of adult uncles and aunts in white coats. Returning to atoms, we all know that they are the basic building blocks of everything that surrounds us, including us. The cement that binds the atoms together is charged particles (nuclei or electrons). Different substances are formed due to different types of interaction (bonding) of electrons. Scientists from Nagoya University (Japan) found that cesium tungsten oxide (CsW 2 O 6) demonstrates an unusual electron binding, which was previously found exclusively in trihydrogen ions, which can be found in interstellar space. How does this electron bond affect the properties of the material, what is its uniqueness, and what does this mean for future research in the field of materials science? We will find answers to these questions in the report of scientists. Go.
Research basis
The authors of this work note that understanding the phase transitions of crystalline solids is one of the main problems in materials science. This includes electronic phase transitions in compounds of transition metals with pyrochlore * structures, consisting of three-dimensional networks of tetrahedra.
Pyrochlor * is a mineral from the class of oxides and hydroxides, which is a complex oxide of sodium, calcium and niobium with additional anions. The pyrochlore formula looks like this: (NaCa) 2 Nb 2 O 6 (OH, F).As an example, scientists cite magnetite Fe 3 O 4 , which exhibits a metal-insulator * transition , accompanied by Fe charge ordering at 119 K, called the Verwey transition * .
The metal-dielectric transition * means that the substance under certain conditions exhibits the properties of a metal (for example, conductivity), and under other conditions, the properties of an insulator.
The Verwey * transition is an electronically ordered phase transition that occurs in a mixed valence system and leads to the ordering of formal valence states in the low-temperature phase.There is still no complete understanding of this transition, although many studies and experiments have been carried out. Nevertheless, the scientific community pays more and more attention to the study of metal-insulator transitions accompanied by magnetic ordering "all-in-one" in 5d-oxides (for example, Cd 2 Os 2 O 7 and Nd 2 Ir 2 O 7 ). The main reason for the popularity of such transitions is the appearance of ferroic ordering of extended magnetic octapoles and the formation of Weyl * fermions in solids.
* — 1/2.In this study, the scientists describe the self-organization of 5d electrons during the electronic phase transition of β-pyrochlore oxide CsW 2 O 6 , found in high-quality single crystals. It was previously reported that CsW 2 O 6 has a cubic lattice with the space group Fd3m at room temperature. In this case, the W atoms form a pyrochlore structure and have a valence of 5.5+ with an electronic configuration of 5d 0.5 . Measurement of the electrical resistivity of polycrystalline samples showed that the metal-dielectric transition occurs at a temperature of 210 K (-63.15 ° C).
* — . ( ), (, , -, ), ( ), , .
It was also previously reported that the crystal structure of the dielectric phase has the orthorhombic space group Pnma . However, theoretical studies have shown that this is not true. Calculations of the electronic structure of the Fd3m phase have shown that there is a strong influence of the Fermi surfaces, which causes a decrease in symmetry to the space group P4 1 32 .
* Pnma , Fd3m and others refer to crystallographic symmetry groups, which describe all possible symmetries of an infinite number of points periodically located in three-dimensional space. More detailed information regarding crystallographic groups can be found here .Recent photoemission experiments with thin films of samples have shown that the valence of W in the dielectric phase is disproportionate to 5+ and 6+.
Research results
To begin with, it is worth considering the phase transition that took place at a temperature of 215 K.
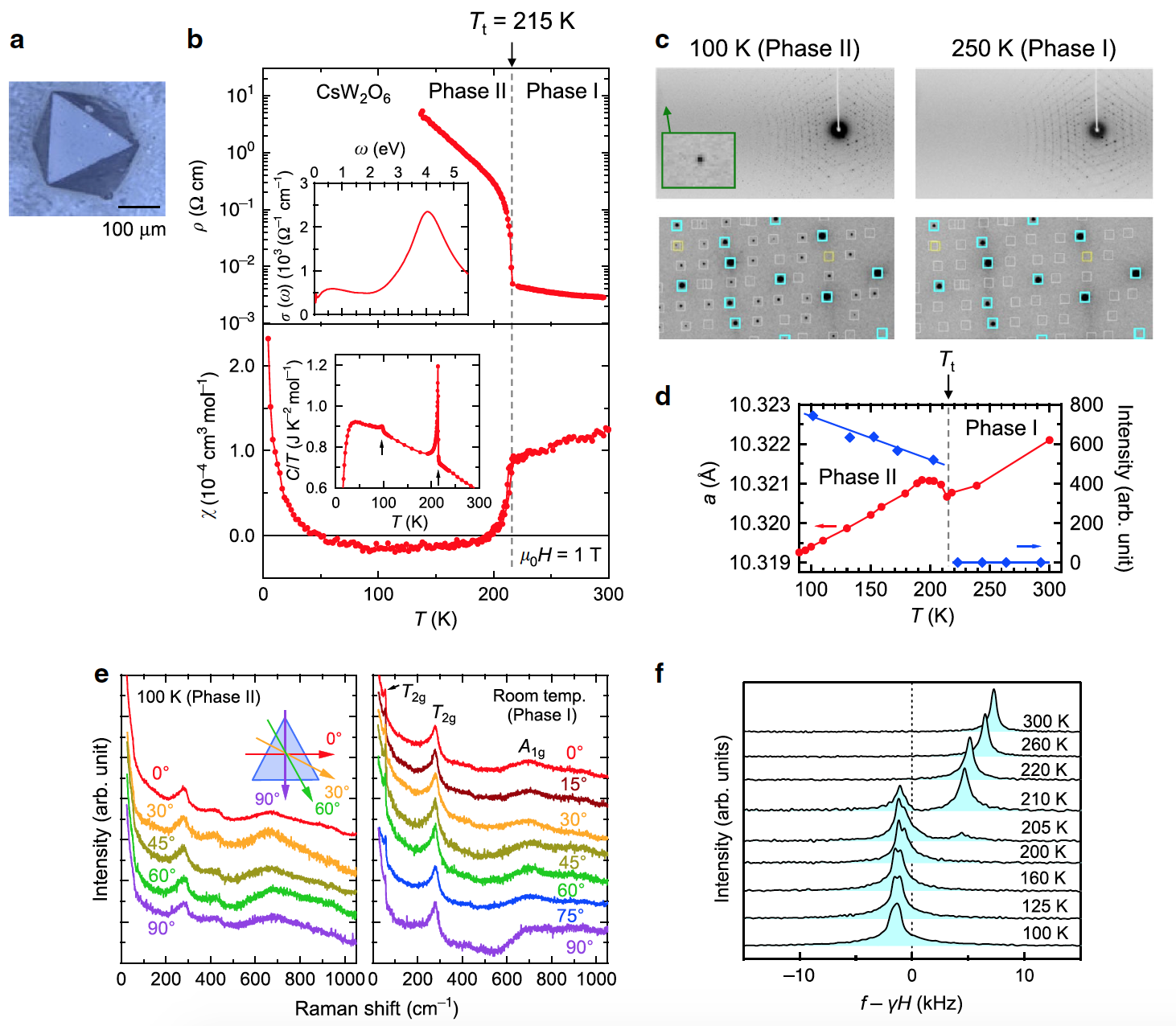
Image №1
Single crystals of CsW 2 O 6 ( 1 ) and W-deficient CsW1.835O6 were prepared in a quartz tube . Figure 1b shows that the resistivity ( p ) of the CsW 2 O 6 single crystal increases strongly when the temperature drops below the T t = 215 K mark , which was also observed in the case of polycrystalline samples and thin films.
This increase in resistance is accompanied by a small but obvious temperature hysteresis. This indicates that the first-order phase transition occurs precisely at Tt (i.e. at 215 K). In this study, phases above and below T t are referred to as phase I and phase II, respectively.
The magnetic susceptibility (χ) strongly decreases below T t ( 1b ), which is also identical to the polycrystalline sample. However, the linear width of the 133 Cs-NMR spectra in phase II does not show any significant broadening compared to phase I ( 1f ). It follows from this that the decrease in χ in phase II is not caused by antiferromagnetic ordering.
Image 1c shows X-ray diffraction patterns of a single crystal CsW 2 O 6obtained at 250 K (phase I) and 100 K (phase II). Each of the diffraction spots at 250 K was indexed based on a cubic cell a = 10.321023 (7) Å with the space group Fd3m , in accordance with previous studies. More diffraction spots appear in the diffraction pattern at 100 K. All of them were indexed based on the cubic space group P2 1 3 with a lattice constant a = 10.319398 (6) Å, which is almost identical to a of phase I. A similar change in diffraction spots occurs at T t , as can be seen from the temperature dependence of the intensity ( 1d ).
It is also worth noting that in phase II the diffraction spots are not divided into several spots and do not change their shape even in the region of a high angle ( 1c ). The Laue class * and the crystal system determined by the observed reflections clearly indicate that the structural change, which retains cubic symmetry, occurs at T t , while phase II is of the m3 Laue class .
The Laue * classes are a crystallographic symmetry class that has a center of symmetry. Of the 32 classes, only 11 are considered Laue classes. The m3 class is a ditrigonal pyramidal system.As can be seen from the polarization dependence of the Raman spectra of the (111) surface, measured at 100 K (phase II) and room temperature (phase I at 1e ), the spectra of phase II are independent of the polarization angle, as in phase I. This indicates the presence of threefold rotational symmetry perpendicular to (111), which is consistent with the assumed cubic symmetry.
These results mean that the Pnma structural model proposed based on powder diffraction data * is incorrect.
Powder X-ray diffraction * - a method of studying a substance by diffraction of X-rays on a sample in the form of a powder.An additional confirmation of the error of Pnma is the fact that this model has a pseudotetragonal distortion of about 0.03%, but this was not observed in this study.
In the polycrystalline CsW 2 O 6 sample, the W-deficient CsW 1.835 O 6 always exists as an impurity phase. Scientists believe that in the process of determining the nature of phase II, an important role was played by the fact that single crystals of CsW 2 O 6 and W-deficient CsW 1.835 O 6 were obtained separately, and measurements of diffraction and physical properties were carried out on single crystals.
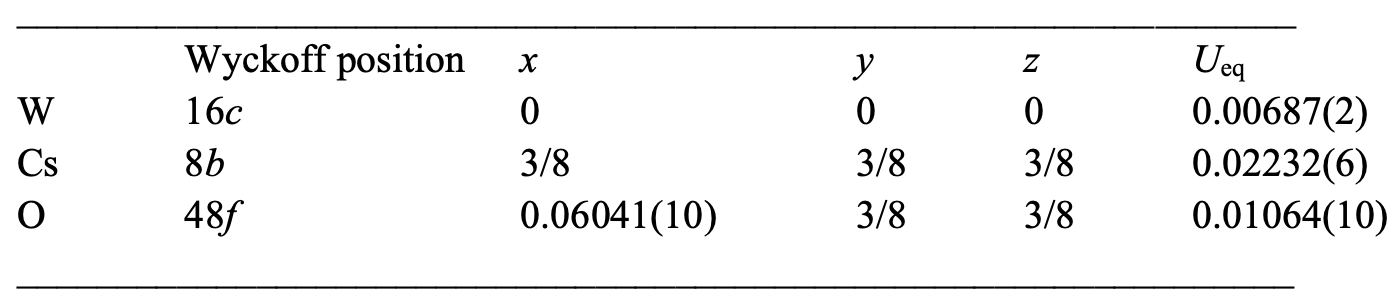
Table 1: CsW crystallographic data2 O 6 phase I (250 K).
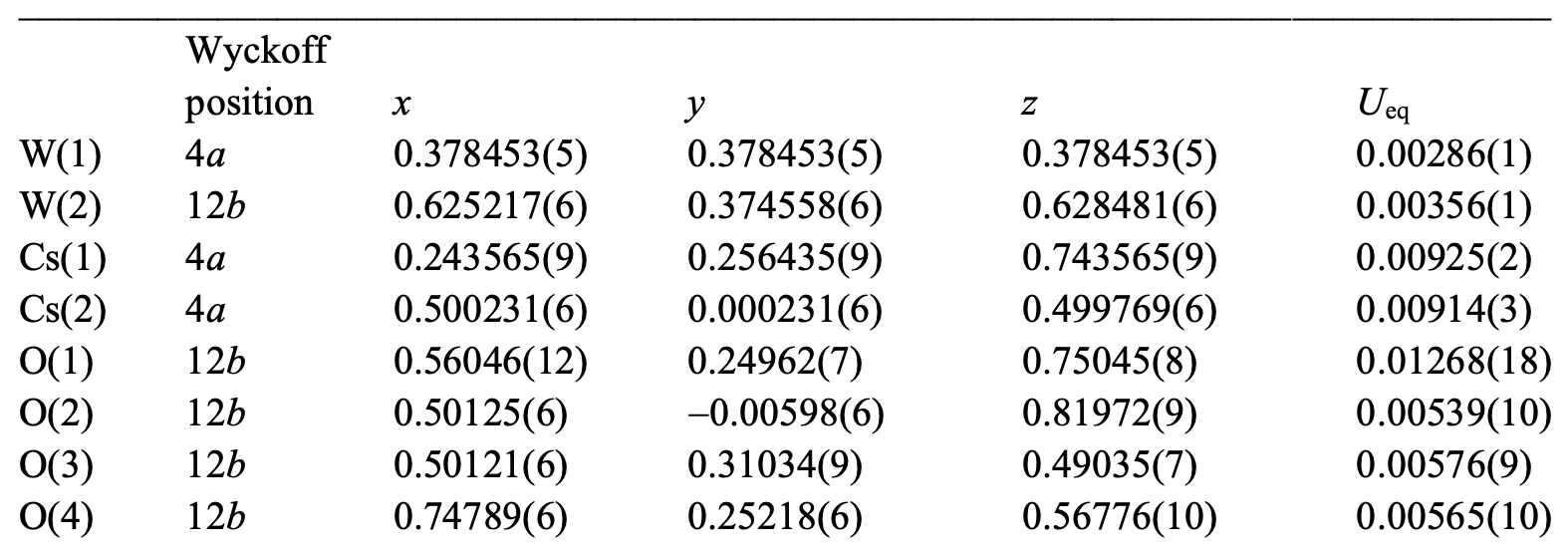
Table 2: crystallographic data of CsW 2 O 6 phase II (100 K).
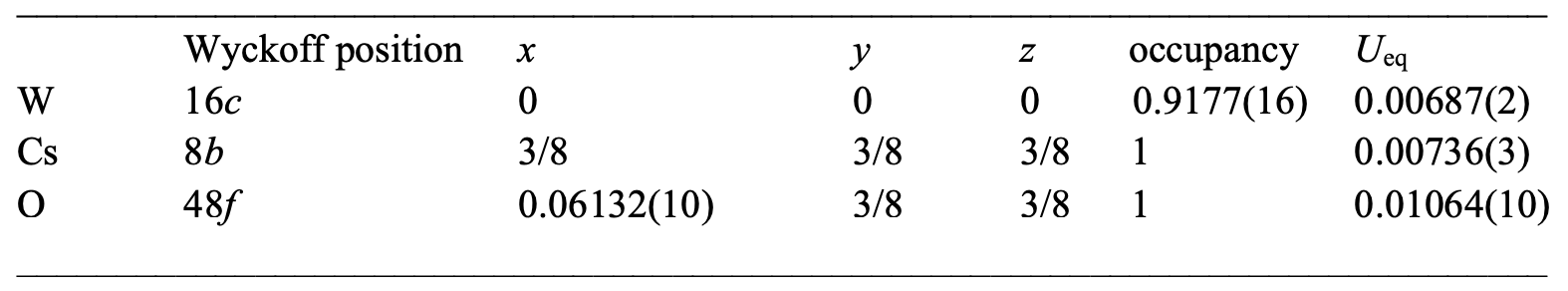
Table 3: crystallographic data for CsW 1.835 O 6 (30 K).
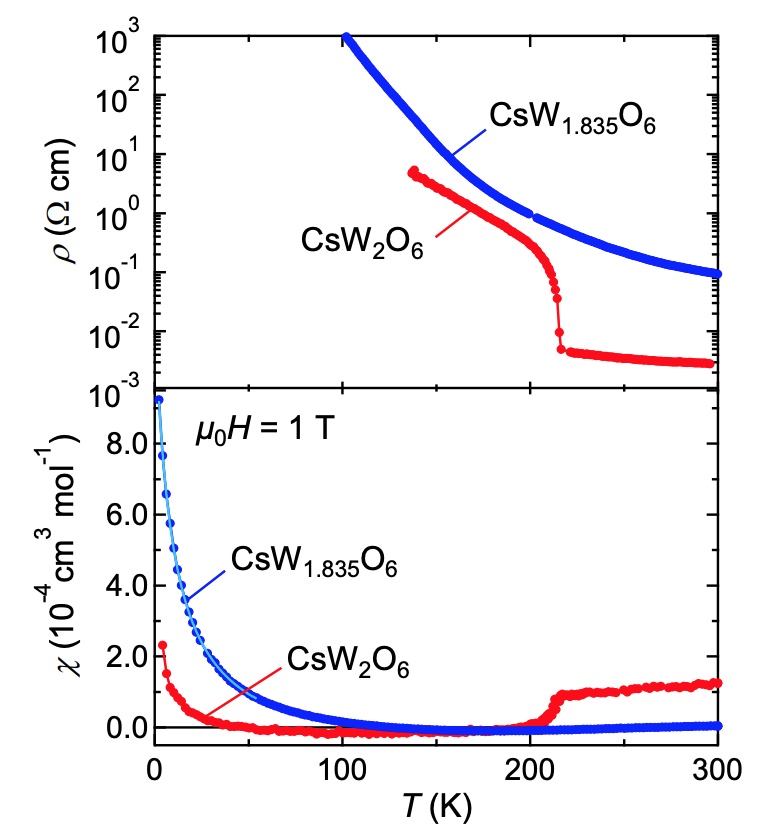
Temperature dependence of the resistance (top) and magnetic susceptibility (bottom) of CsW 1.835 O 6 single crystals .
In the next phase of the study, the scientists took a closer look at the crystal structure of phase II.
In phase I with the space group Fd3m, each of the Cs, W, and O atoms occupies one region, where the Cs and W atoms form the structures of diamond and pyrochlore, respectively ( 2a ).
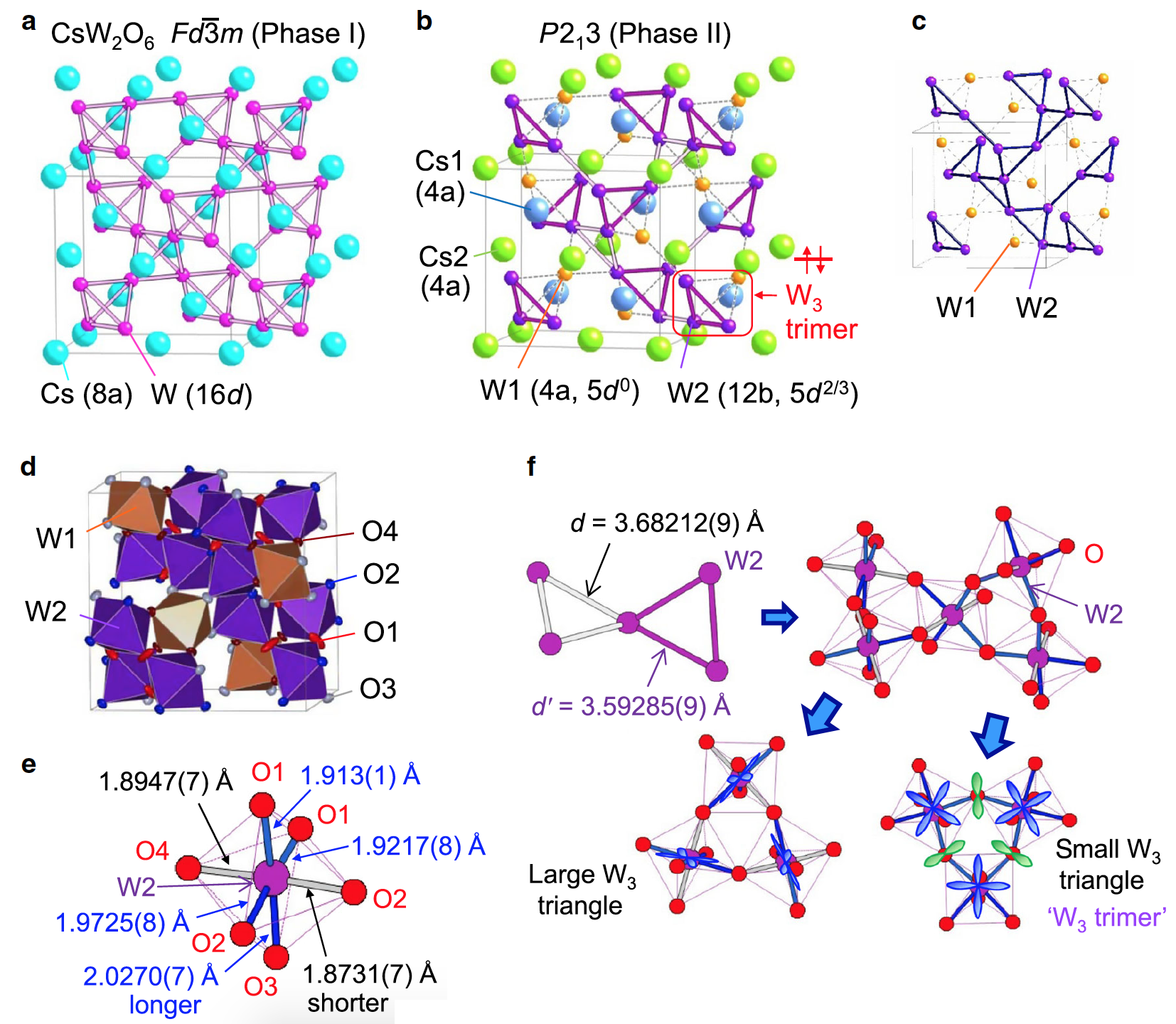
Image # 2
In phase II with the space group P2 1 3 , the Cs atoms occupy two different centers and form the "sphalerite" structure (named after the mineral of the same name, also called "zinc blende * ") ( 2b ).
Deception * refers to minerals that are not metallic ores, but have a semi-metallic luster and other features (color, density) inherent in both metal ores and minerals.This was further confirmed by two peaks in the 133 Cs-NMR spectra corresponding to two Cs regions, which appear as a slight splitting of the peaks at 200, 160 and 125 K ( 1f ).
On the other hand, W atoms occupy two portions with a ratio of 1: 3 in phase II ( 2b and 2c ), which is incompatible with the charge ordering W 5+ - W 6+ atoms W 5+ , and W 6+ in the ratio 1: 1.
According to the calculation of the bond valence sum for the W - O distances determined from the X-ray structural analysis of a single crystal, the valences of the W (1) and W (2) atoms were 6.07 (3) and 5.79 (3) at 100 K (phase II), respectively.
Considering that the parameters of the valence sum of a reliable W 6+ bond are available, but the parameters of W 5+ are not, it is logical that the W (1) atoms are W 6+ without 5d electrons. In this case, the valence of the W (2) atoms becomes equal to 5.33+ with 5d 2/3 electronic configurations .
It follows from the above calculations that charge ordering with noninteger valence takes place at T t . In fact, single crystals of W-deficient CsW 1.835 O 6 , where all W atoms have valence 6+ without 5d electrons, do not show a transition at T t .
In phase II, the W (2) atoms form a three-dimensional network of small and large regular triangles, which are alternately connected to each other by common angles ( 2b ). Although the difference in size between the large and small triangles is about 2%, the arrangement of the occupied 5d orbitals between them is completely different, which leads to the formation of the W 3 trimer in the small triangle. If there were no alternation of triangles W 3 , the sublattice W would have a hyperkagomny (three-dimensional structure of connected triangles) structure ( 2c ). The presence of alternation indicates that a "breathing hyperkagoma" structure (ie, with gaps, as opposed to a uniform hyperkagoma) is formed during phase II.
Charge ordering in phase II CsW 2O 6 curiously, the "Anderson condition" is supported in an unusual way. Anderson said that magnetite has an infinite number of charge ordering models, when all tetrahedra in the pyrochlore structure have the same total charge (this is Anderson's condition), and this macroscopic degeneracy strongly suppresses the Verwey transition temperature.
Nevertheless, there is evidence that not only magnetite, but also other mixed valence pyrochlore systems, such as CuIr 2 S 4 and AlV 2 O 4, demonstrate an ordering of charges that violates the Anderson condition. In this case, the energy obtained due to the σ-bond between the d-orbitals of neighboring atoms must be large enough to compensate for the loss of Coulomb energy due to violation of the Anderson condition.
But in the case of CsW 2 O 6, the situation is different. Its charge ordering satisfies the Anderson condition, where each tetrahedron consists of three W 5.33+ atoms and one W 6+ atom . However, this ordering format differs from the one proposed by Anderson and Verwey, where the valencies were integer with a 1: 1 ratio.
Hyperkagome type ordering often appears in pyrochlore systems with a ratio of two types of atoms of 1: 3. Thus, CsW2 O 6 is currently the only example of an ordering of hyperkagom type with a nontrivial nature of formation.
The quite expected question arises - why exactly such an ordering format appears in CsW 2 O 6 ? According to scientists, the answer can be obtained by taking a closer look at the instability of the Fermi surface of the electronic band structure of phase I, i.e. understand the movement and interaction of electrons in this phase.
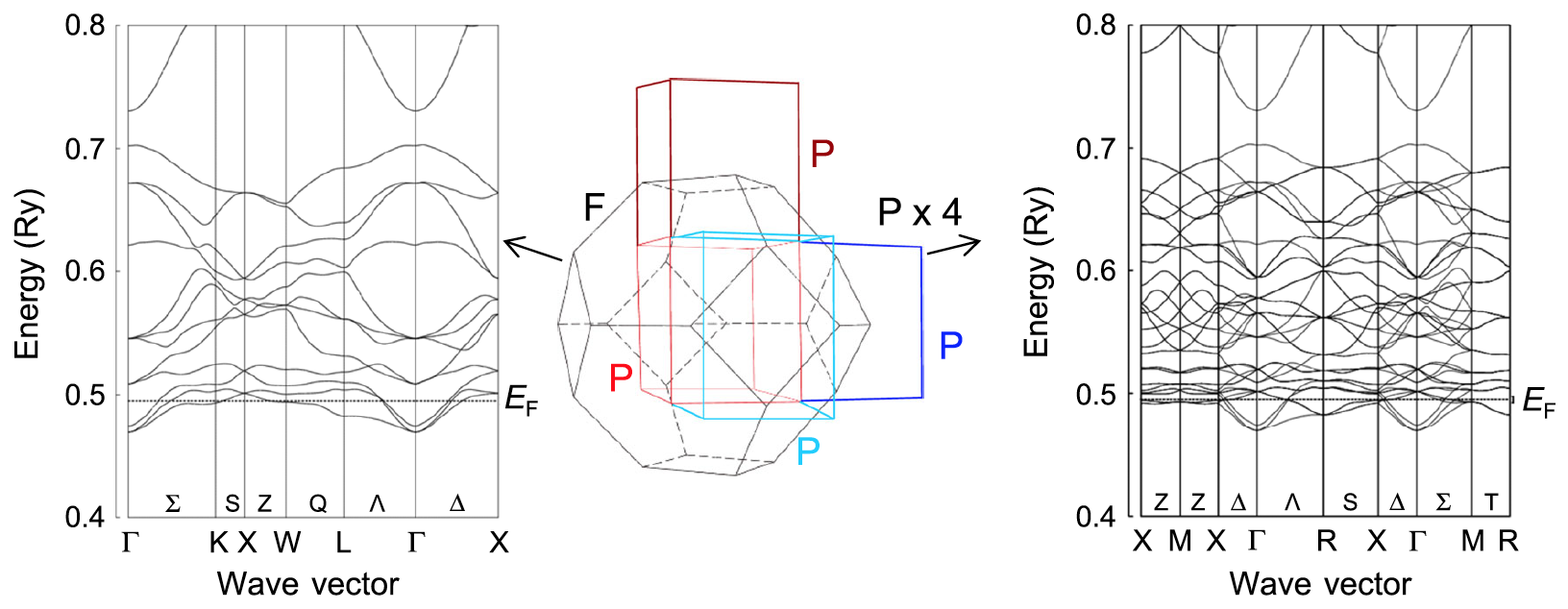
Image # 3 The
above image shows the band structure of phase I on the left, and the overlapping band structures on the right, obtained after a parallel shift of the electron bands corresponding to the change of a primitive cell from a face-centered system to a simple system.
Cubic system (from left to right): simple, body-centered, and face-centered.
As seen on the right side of image # 3, band crossing occurs near all points where the electron bands touch the Fermi energy (E F ). Consequently, the Fermi surfaces are well nested due to the parallel shifts of the electron bands, corresponding to the loss of centering operations.
Scientists call this scenario of the development of events "three-dimensional nesting". This means that a lot of electronic energy is generated by structural changes associated with the aforementioned symmetry change. Therefore, this three-dimensional nesting can be an important component of the transition at 215 K.
If this effect is considered as the only driving force in the onset of the transition, then a structural change should occur from Fd3m to P4 1 32 or P4 3 32 , which was already expressed in an earlier theoretical study. In this case, the W (2) atoms should form a homogeneous hyperkagomic structure. It is also assumed that the band gap does not open at the Fermi energy in the cases of P4 1 32 and P4 3 32 , which is inconsistent with the dielectric nature of phase II observed in this study.
In reality, the phase II space group is P2 13 , which is a subgroup of P4 1 32 and P4 3 32 , and the W (2) atoms form a breathing hyperkagomic structure, where the size of a small triangle is 2% smaller than that of a large one.
In addition, the orientation of the occupied 5d orbitals is important to lower the symmetry from P4 1 32 / P4 3 32 (uniform hyperkag) to P213 (breathing hyperkag). For octahedron W (2) O6 phase II ( 2e ) apical two communication W (2) -O (marked gray) 3-8% shorter than the other four equatorial bonds (marked in blue). This suggests that the octahedron is uniaxially compressed.
Such a distortion, according to scientists, strongly resembles the classic example of the Jahn-Teller effect * in electronic systems t 2g . In this case, the 5d orbitals lying in the equatorial plane should be occupied by electrons ( 2f ).
The Jahn-Teller effect * - occurs when the interaction between electrons and vibrations of nuclei leads to the formation of local deformations and a change in the crystal symmetry (static effect), or when vibronic states are formed (dynamic effect).There is a significant overlap between the occupied 5d orbitals in the small triangle through the 2p orbital O. But in the large triangle, there is a slight overlap. This indicates that two electrons in three W (2) atoms are trapped in the W 3 trimmer in a small triangle.
For the formation of this trimer, the electron correlation of 5d electrons in CsW 2 O 6 can be another significant factor. In the CsW 2 O 6 trimmer, two 5d electrons form a spin-singlet pair, which leads to a non-magnetic and dielectric ground state. Thus, we observe an alternative type of self-organization of d-electrons, realized in a strongly correlated 5d-oxide.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists and additional materials to it.
Epilogue
The result of this study was the discovery that trimers of the regular triangle W 3 are formed during the 215 K transition in β-pyrochlore of the oxide CsW 2 O 6 . This was determined by measuring the structural and electronic properties of single-crystal samples.
In fact, the scientists discovered tritungsten molecules in CsW 2 O 6 single crystals cooled to -58 ° C. At room temperature, CsW 2 O 6 is a good conductor, but becomes an insulator when cooled.
When a crystal is in a conductive state, tungsten molecules form three-dimensional networks of tetrahedral pyramids connected at their corners, known as a pyrochlore structure. And electrons symmetrically distributed between molecules form their bond. If the sample is cooled, then the electrons change their position, from which two types of tungsten atoms appear, which differ in their valence. Such changes lead to a distortion of the bond of tungsten with oxygen atoms, which leads to a more compressed form of the compound.
During all of these perturbations, the lower valence tungsten atoms form small and large triangles on the sides of the tungsten tetrahedra, with very small tungsten molecules forming small triangles. The three tungsten atoms, which are the tops of these triangles, are held together by just two electrons.
Scientists say that at the moment CsW 2 O 6 is the only known example where such a bond format (two electrons per three atoms) manifests itself as a phase transition. In subsequent works, the authors of this study intend to study compounds with pyrochlore structures in greater depth, which will make it possible to discover new materials with extremely unusual properties.
Thanks for your attention, stay curious and have a good work week, guys. :)
A bit of advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting content? Support us by placing an order or recommending to friends, cloud VPS for developers from $ 4.99 , a unique analogue of entry-level servers that we have invented for you: The Whole Truth About VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server correctly? (options available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Is Dell R730xd 2x cheaper in Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - From $ 99! Read about How to build the infrastructure of bldg. class with the use of Dell R730xd E5-2650 v4 servers at a cost of 9000 euros for a penny?