
In general, today we will not talk about girlish tears, the article today is about irritants, lacrimators and tear gases. The topic is important, and at least once "on the farm" will come in handy. For those who need emergency help and do not have time to immerse themselves in theory - go straight to the conclusions with recommendations .
Intro
The letter came to me with something like this:
. 3 , . , <...> , ( paro cardio-respiratorio, , 3 <...> Condor ( ). chlorobenzylidenemalononitrile clorbenzilideno malonitrilo. , Condor. <..> 5 . . ?
Chile's beautiful streets ...
Although we are not Chile, the topic may be relevant for various reasons, from a hooligan who sprayed gas in a trolleybus, to “blind treatment” of passers-by during the civil protests taking place nearby. And as you know, forewarned = forearmed.
So the problem exists. The task is to figure out how to deal with it, preferably with little blood. First, I'll start with the definitions. Any tear gases are classified as chemical warfare agents (which means that protection from them falls under my specialization as a "rhbz fighter") and constitute a group of so-called. irritants(from Lat. irritantis "irritating"). Substances of this group, when exposed to a person, cause severe local irritation of the mucous membranes, skin and nerve receptors located in them with the formation of a reflexive protective reaction of the body aimed at eliminating the irritating substance (itching, burning, pain, perspiration, lacrimation, rhinorrhea, sneezing, cough ). They are divided into 2 main groups:
- sternites are substances that cause uncontrolled sneezing and coughing.
- lacrimators - substances that cause profuse lacrimation;
Sternites (from ancient Greek στέρνον - chest) are a group of toxic substances that irritate the respiratory system and cause sneezing, coughing, chest pain, severe and uncontrollable vomiting. Typical examples are adamsite (diphenylaminechloroarsine), diphenylchloroarsine, diphenylcyanarsine. For the most part, these are all arsenic compounds and they are universally prohibited by international conventions. Technically, this group also includes such a lacrimator as CS-gas. By the way, the sternites were often called the "blue cross" (German: Blaukreuz), because during the First World War, if there was an irritant inside the shell, a blue cross was put on the shell, if the suffocating gas was a green cross. In some museums you can still see ...

In the modern world, the task of destroying manpower (especially within the framework of a single country) is not worth it, more often the task is to temporarily disable and render harmless. Especially when it comes to protests. In the United States, for example, the name “riot control agents” (RCA) has even been coined for chemicals with a neutralizing effect, while in our country, in the old fashion, it is an irritant and an irritant. In general, tears are used to disperse crowds or cause temporary disability for members. It is also worth noting that tears are prohibited for use in hostilities (Article I.5 of the Chemical Weapons Convention), but at the same time their use by civilian law enforcement agencies is permitted (Article II.9 of the Chemical Weapons Convention).And since gas canisters for self-defense (small "civilian" copies of service special equipment) contain the same components, but in smaller quantities / concentrations, everything written about "big brother" applies to "little brother".
How it all works
Irritants are so-called. peripheral sensory stimuli.
how they act in terms of neurology
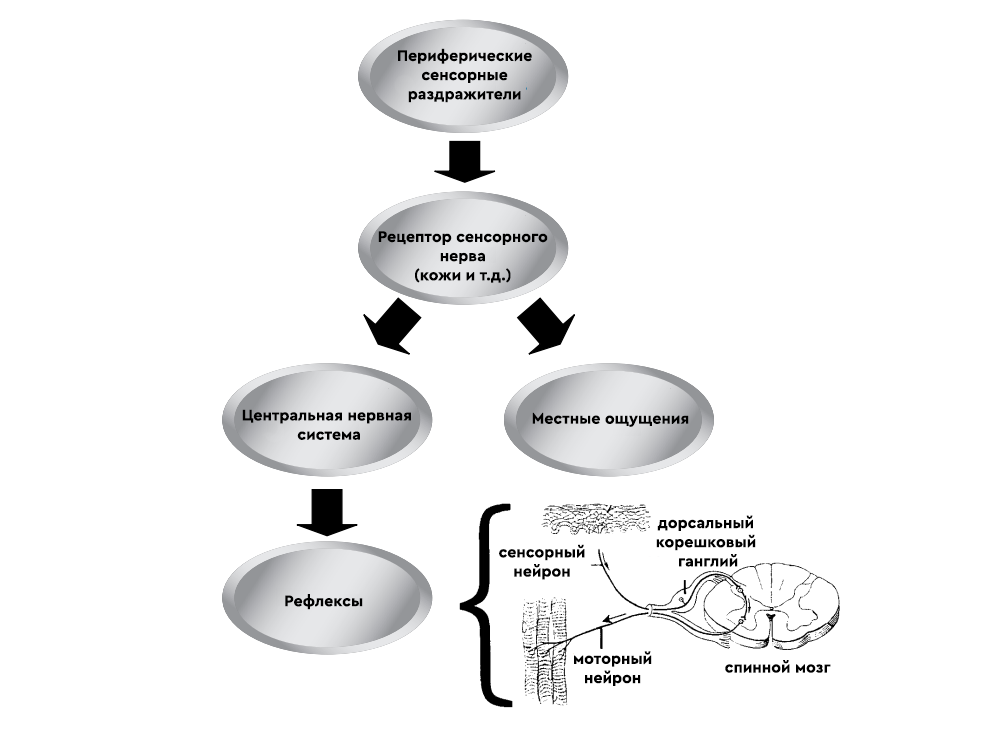

It interacts locally with receptors on sensory nerves in the skin, eyes, and other mucous membranes, causing severe pain and irritation. Mechanical, thermal and painful inflammatory signals are transmitted via cationic channels (TRP). In fact, these are chemical sensors present in the plasma membranes of the mucous membranes of humans and mammals (
Remark about bacon and mustard
, TRPA1 . , — . , , - «» . , , , ? :) , -, . , . ( ). … , :) , « » — papite
, — , , , . - , — , , , , . , , II . , , .
, — , , , . - , — , , , , . , , II . , , .
Most often, substances that activate TRPA1 have the so-called. electrophilic carbon, which can undergo a reversible nucleophilic attack by a thiol (sulfur-containing) cysteine amino acid fragment or a lysine amino group present at the active site of the TRPA1 receptor.
By the way, this receptor is not so simple as it seems. The picture below shows the genesis of ideas about the role of receptors in human sensory perception. And if in the traditional view (A) it was believed that TRPA1 is responsible only for interaction with mustard oil, then in modern views it is already trying to ascribe even a reaction to cold:
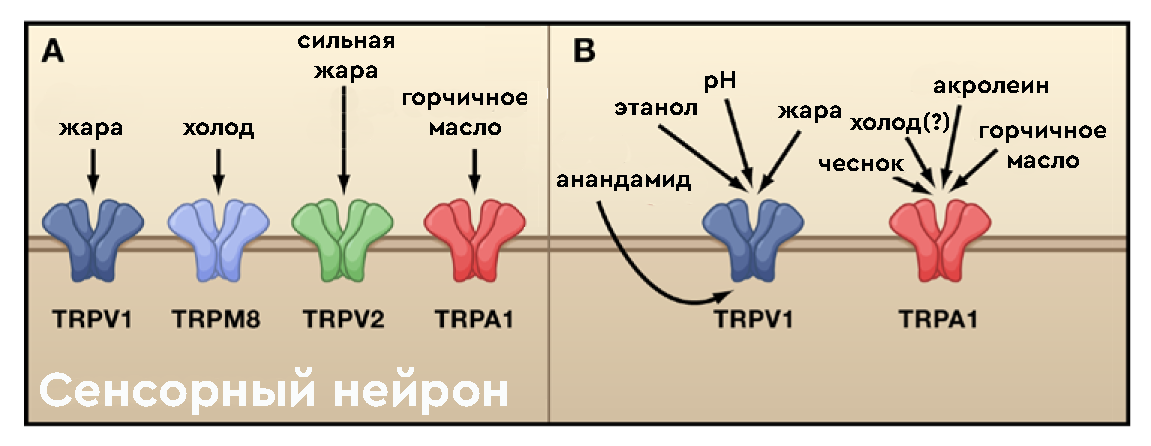
It is logical to assume that antagonists, i.e. molecules that prevent the above channels from opening will reduce the effectiveness of all kinds of lacrimators, mustard oil and ... even cold (?). Such substances (= antidotes to tear gas) exist, but so far in the form of concepts and all kinds of substances that have not passed clinical trials. For example, we can mention endogenous resolvins D1 and D2, maresin . Of the chemical reagents, the most understandable is the dye ruthenium red used in biochemistry (although it should be noted that it is not selective and blocks a bunch of other useful receptors along with TRPA1):
Buy in China


The principle of operation of the anta gonist is illustrated in the figure (yellow balls are those substances that open the ion channels, they are also called a gonists).

Classification of compounds of lacrimators
Having outlined what works and why, it's time to move on to the main representatives of the lacrimator family. Here I will immediately note that all these substances, without exception, are NOT GASES (although they are constantly called that). These are solids and liquids, the main method of delivery of which to the body of a peaceful protester is through an aerosol form. Often only the method of aerosol production is varied, through "spray" or through "thermal sublimation", just like in a mosquito fumigator .
About 15 types of various tear gases have been developed in the world, but many of them long ago became the property of history because of their properties of chemical warfare agents (more precisely, not properties, but long-term health effects, such as carcinogenicity, etc.).
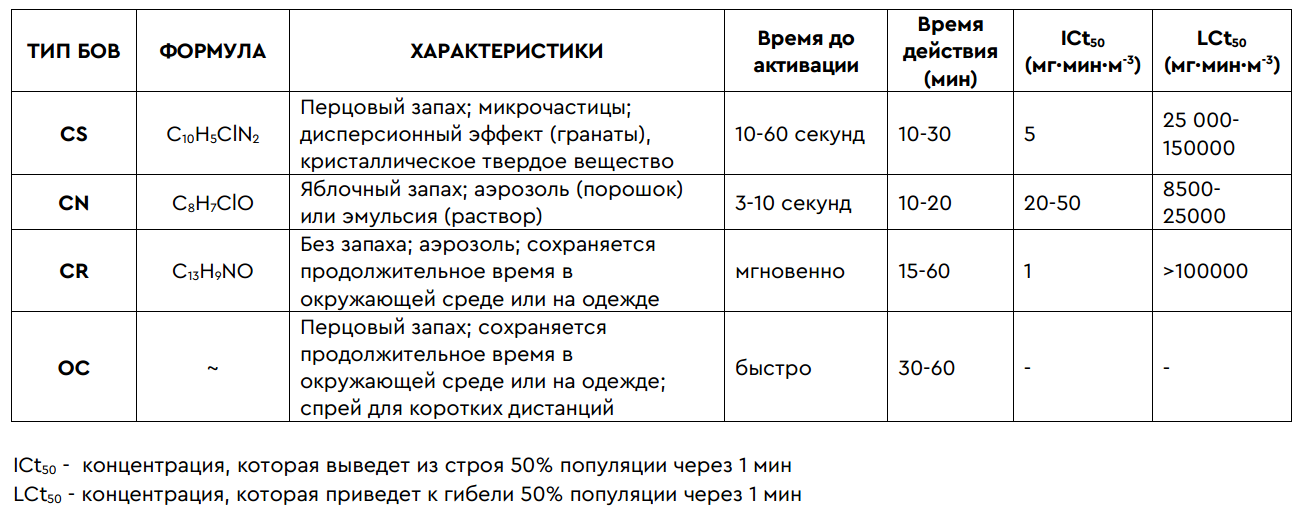
Among the long list of substances, the most important, due to their effectiveness and low risk in use, are the CN / CS / CR / OC “gases”. Although the risks are low for a healthy person, sometimes in the presence of chronic diseases (in particular, problems with vision or respiratory organs), they can seriously increase.
In general, the effects of tear gas can lead to numerous short-term and long-term health effects: the development of respiratory diseases, serious eye injuries and related diseases (traumatic visual neuropathy, keratitis, glaucoma and cataracts), dermatitis, cardiovascular and gastrointestinal damage. intestinal systems. Death is also possible, especially in cases of exposure to high concentrations of tear gas or the use of tear gas in enclosed spaces. In addition, when using fired cartridges, there is always a risk of this blank falling into a person (= bruises, abrasions and even fractures). Although the medical effects of the gases themselves are usually limited to mild skin inflammation, delayed complications are possible. The table can help to add an approximate impression:
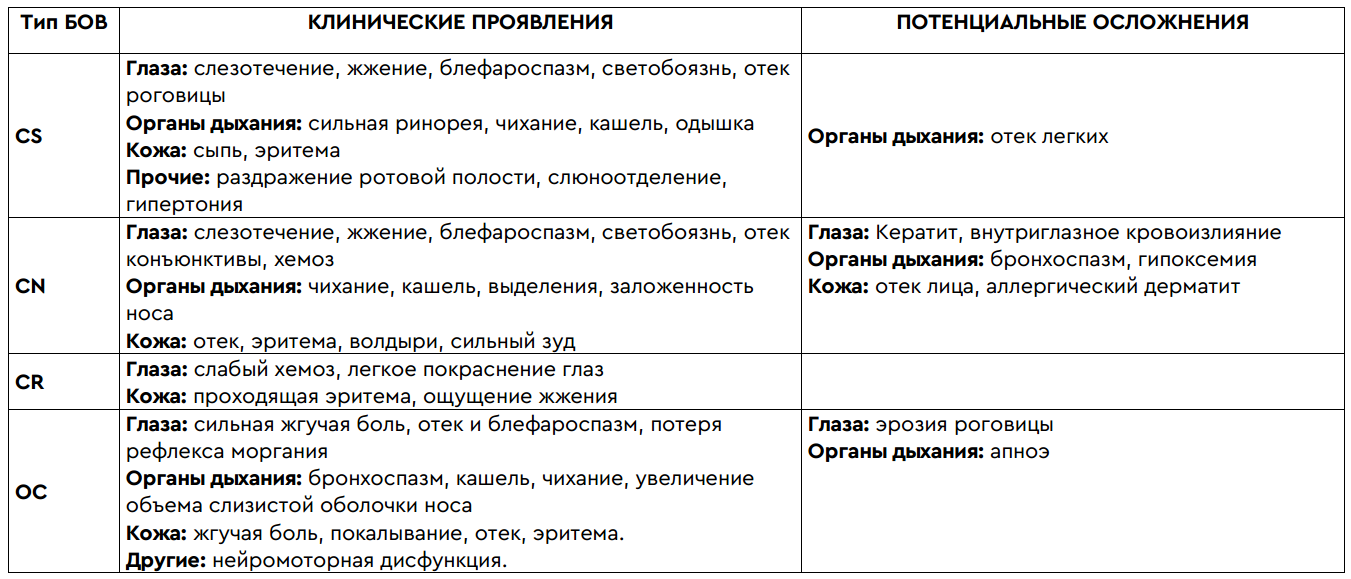
Having described the possible consequences, you can safely proceed to a brief overview of existing and actively used irritants in the modern world. I'll start with the lacrimator, which was the first - with the so-called. "CN gas"
CN-gas (aka chloroacetophenone , aka bird cherry gas, aka lithin, phenacylchloride , orlit, substance No. 34, R-14) = CAS 532-27-4

Chloroacetophenone can be called an old-timer in the field of tear poisonous substances. It was first obtained during the First World War, but found active use only in the middle of the 20th century. CN is a white solid with m.p. 54-56 ° C, bp 245 ° C. This substance got its start in life in the 60s of the last century, when the American chemist A. Litman, responding to the robbery of one of his wife's friends, developed the concept of a gas cartridge with CN. The Litman manufactory was soon sold to Smith & Wesson and began to produce the so-called. "Mace" (eng. Mace) for the fight against law and order violators. In addition to 1% chloroacetophenone, the composition included solvents 2-butanol, cyclohexene, and propylene glycol.By the early 90s, the formula was being optimized into a complex lacrimator with marker functions (= CN + pepper extract + UV fluorescent dye). CN acts on TRPA1 receptors. Chloroacetophenone is stable, does not decompose when heated (= can be used with "fumigator" pomegranates). Loses its lacrimator effect rather quickly, due to reversible condensation in the air shortly after dispersion. The maximum concentration at which the aerosol in the air is stable - about 4.5 mg / m³ of air, higher - begins to quickly "crumble". Slightly soluble in water, readily soluble in chloroform and other organic solvents. Today, due to many toxic effects (including death from suffocation and damage to the lungs) and strong blistering action (= on contact with the skin ~ 0,5 mg of a substance in tens of minutes, a burn is formed with the formation of blisters) is completely replaced by other tear substances.
UPD . In the table with the characteristics of gases for CN, the "apple smell" is indicated, but withSergeyMaxI will clarify “Chloroacetophenone (CN) smells distinctly of bird cherry, not apple. Actually, this is where its old name is "Cherry". Honestly, my main sources of information are foreign, and they probably have such a shrub that is not very common, here is the "apple smell". Let there be two options, who likes what more.
NB Soviet textbooks on GO recommended decontamination on CN surfaces with heated aqueous-alcoholic solutions of sodium sulfide . The skin can be washed off with a strong jet of water or with a 5% baking soda solution (NaHCO3). Strong oxidants (sodium hypochlorite, sodium permanganate) oxidize CN to harmless benzoic acid. Eyes — rinse abundantly with saline.
CR-gas (aka dibenzoxazepine , aka DBO, aka Algogen) = CAS 257-07-8

Dibenzoxazepine was developed in the 1950s and 1960s in the UK.
UPD . clarification fromgeher, about the fact that CR is used in BAM cartridges for a metered aerosol spraying device , in gas cylinders "Tarantula" and "Slezinka" (in combination with IPC). Clarification fromsvchsechen "The OC + CR mixture is used in the Tekhkrim gas cylinder" Kortik "
NB The best way to remove from the skin is to wipe with an alcohol-soaked tissue or rinse with a large volume of water and soapy foam. CR reacts with strong oxidants (hydrogen peroxide, chloramines, hypochlorites, sodium permanganate) to form non-irritating products. Eyes — rinse abundantly with saline.
CS-gas (aka chlorobenzalmalonodinitrile, aka "Lilac gas", aka P-65) = CAS 2698-41-1

S is one of the most widespread lacrimators in the world (both for official special equipment and in civil self-defense). Most of the "grenades" that journalists show when commenting on protests or riots are equipped with CS. The photo, by the way, is an example of the same expired Brazilian gas that was used in Chile. It is a white solid with so pl. 93-95 ° C, bp 310-315 ° C. In fired cartridges, the pyrotechnic composition heats up the CS powder and makes it actively evaporate. Protesters in Hong Kong and Turkey tried to more or less successfully neutralize the sublimation process by submerging grenade cartridges in water (note mine - water should be with a pH> 9).
CS activates TRPA1 receptors. With prolonged exposure to the skin, CS can cause burns, leaving scars. Garments that have been exposed to CS gas must be washed several times. People or objects contaminated with CS gas can cause secondary exposure to those around them by re-dispersing the lacrimator. The substance itself is quite stable, but upon thermal degradation, it can form toxic products, so some studies recommend replacing CS with the more irritating CR. Dichloromethane can be used as a solventand methyl isobutyl ketone (MIBK), which are inhalation toxic compounds. CS is soluble in acetone, moderately soluble in alcohol, slightly soluble in water. At high temperature and humidity, CS exposure increases, but with regular or prolonged exposure, people may develop tolerance.
NB c >9 ( , 5%-10% Na2CO3), ( ; , ; CS , ). — . , . « » , , .. , . CS - (NaOH/KOH).
OC ( oleoresin capsicum, , ) = CAS 8023-77-6

And finally, our favorite, pepper extract, which in different variations, in fact, captured the world of both special and civilian lacrimators. In fact, it is an alcoholic (ethanol) extract of chili pepper, the alcohol from which was then evaporated, and the resulting gum-like substance was dispersed in the desired solvent (for example, propylene glycol). The main component of the pepper extract is the alkaloid capsaicin (8-methyl-6-nonenoic acid vanillylamide). This alkaloid is a white crystalline substance (mp 62-65 ° C, bp 210-220 ° C at 0.01 mm Hg) practically insoluble in water and aqueous solutions of alkalis, but easily soluble in organic solvents , ethyl alcohol and fats. Possesses chemical resistance. The pepper extract contains several different capsaicinoids of various strengths.All of them are lipophilic (fat-soluble), colorless, odorless, resinous compounds.
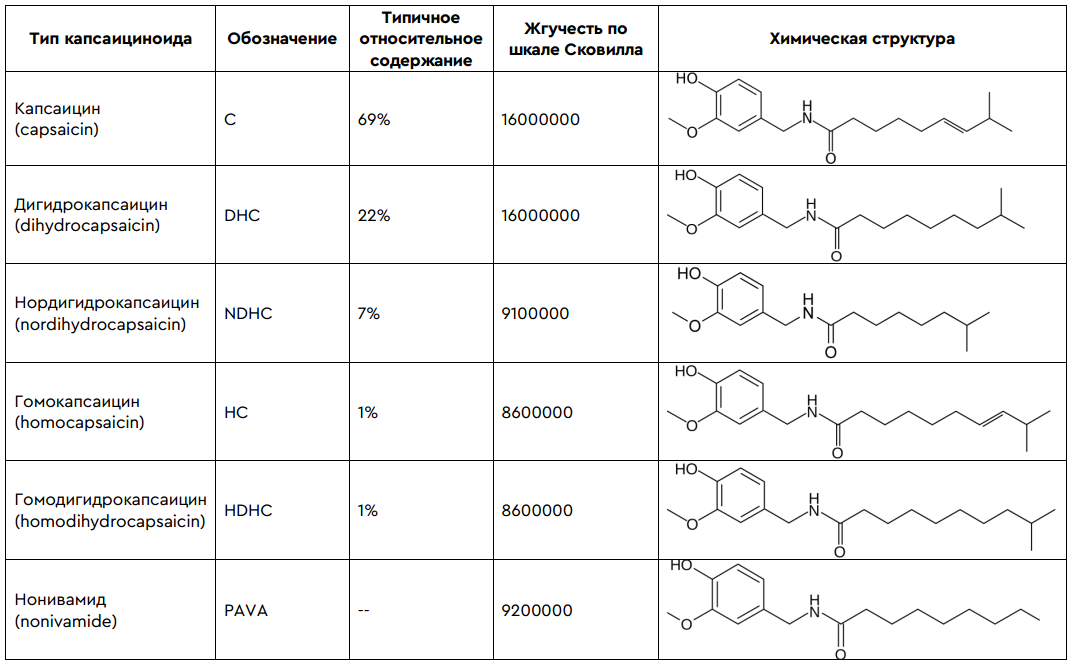
Due to the different pungency of capsaicinoids, it is impossible to accurately determine the effectiveness of gas cartridges from different manufacturers. The capsaicin concentration method does not work because there are 6 capsaicinoids with varying levels of irritation. Most often, they just talk about some kind of concentration, without reference to a specific component. Therefore, if you have already decided to buy the right pepper spray, look at the output of the active substance (DV output per second) and the liquid composition output (ZhS output per second). The first characteristic is responsible for the maximum rigidity of the balloon, and the second for the speed of delivery of the irritant to the target. The higher they are, the better. But I digress, the conversation is about defense, not attack.
Returning to capsaicin, I would like to note that, unlike all other lacrimators, capsaicin / -ny does not act on the usual TRPA1 receptor, but acts on the TRPV1 receptor. This receptor is activated at temperatures above 43 degrees Celsius, pH below 6 and the presence of endogenous lipids. Capsaicin, when applied to the skin (in reasonable amounts) leads to the so-called. heating effect, due to which you can drown out some other stimulating factors. Those. "Pepper extract" is able to relieve pain, due to which it is actively used in medicine in the form of various warming and anti-inflammatory ointments.
Well, in doses exceeding therapeutic ones, pepper extract works as a tear and irritant substance, both individually and in a mixture with some kind of IPC (about it below) or CS. Works great not only against people, but also against dogs and bears, does not work against reptiles (they need CN). By the way, alongside natural capsaicins, a synthetic analogue - nonivamide or PAVA - coexists peacefully. According to the state of aggregation, it is a white powder with so pl. 57 ° C. Alcoholic 0.3% solution of PAVA in special GB is considered even more effective than individual CS. Note that piperine can be an interesting potentially substitute for capsaicin (natural and synthetic), as it, dear, acts on two types of "lacrimator" receptors at once - on the usual TRPA1 and on the "pepper" TRPV1. According to its state of aggregation, it is a yellowish liquid, boiling at 130 ° C.
NB Pepper has its own antidotes - TRPV1 receptor antagonists and capsazepine . As a method of decontamination of the skin , the use of various liquid antacids is recommended , for example, "milk" from burnt magnesia (MgO) with water. In addition, capsaicins on surfaces can be decontaminated with sodium hypochlorite solution (followed by rinsing with soapy water and copious lather). Using various fats to remove pepper extract from the skin is advisable , . ( , ) . , , .
( , N-nonanoylmorpholine, MPK) = CAS 5299-64-9
As a makeweight, I could not help but write about a kind of "local landmark", about the substance of the IPC. During my school years, gas canisters "SHOCK" were distributed in my area (they were often sold for pennies to the Gypsies). In the composition was this same IPC and these means of protection acted in any way. It is not surprising, because for the first time this substance was noticed in the United States back in 1958, but the matter did not advance beyond laboratory research. But in the post-Soviet expanses, the substance has taken root. When used as a solo component, it has low efficiency, even at the maximum allowable concentration. Human volunteers who were exposed to IPC in laboratory conditions immediately experienced a burning sensation in the nose and a sore throat, but all the symptoms disappeared very quickly when exposed to fresh air.For BMD, the irritating effect is more pronounced (close toadamsite ), rather than tearing. On the other hand, the VO2 max is preserved for a long time in environmental objects and is the most persistent compound among irritating substances. Probably due to all of the above, it is used as a co-solvent (and additional irritant) in compositions containing CR / CS. It is believed that such mixtures are effective against dogs and people under the influence of alcohol and drugs. And it's cheaper than raising capsaicin concentrations to unprecedented heights. In the end, I will add that IPC, like many of the substances mentioned in my article, does not dissolve in water, but is soluble in acetone and some other organic solvents. This burning liquid boils at 310 ° C.
Conclusions and recommendations
Well, here we come to the generalization and development of a methodology. With regard to preventive measures, i.e. protection against the effects of tear and irritating substances, then the recommendations here are absolutely identical to the recommendations that were given earlier in the case of a coronavirus pandemic .
"Tear gas" ≠ gas . "Tear gas" = aerosol .
This means that protection against most irritants is identical to protection against any other aerosols, incl. biological, radioactive, etc. etc.

It is necessary to use all the same PPE familiar already in 2020: respirators and half masks with protection class N99 / N100 ~ FFP2 / FFP3 (or if there are fans - gas masks and full face masks with aerosol protection classes P2 / P3), sealed goggles (for example, swimming goggles), dental shields and gloves to protect the skin of the face and hands. Clothes with long sleeves, covering most of the skin surface, will not be superfluous.
2020 is the year under the sign of aerosol
, , - . , , - — . . , ,

, .

, .
If, nevertheless, you managed to get into the zone of infection, then the tactics of action boils down to removing the lacrimator / applying deactivating substances to the mucous membranes and only then, if necessary, reduce the pain effect with anesthetics.
IN CASE OF POISONING WITH LACKS / IRRITANTS, IT IS NECESSARY :
- . . CN/CS/CR-, ( ). /c .
- . . CN/CS/CR, .
- , . , . C , .. , , . , .
, , , . , , (.. « »), . , . « », , . , , / / .
4. :
- CN — 5% ( , NaHCO3). CN « » — - .
- CR — / . , CR, , (= -). , , R 48 .
- S — >9 ( ), , 5%-10% Na2CO3, . CS , , ( - ). , . , ( ) (= -)
- OC/PAVA/MPK — ( , , , ). , « », , (= -). , , «» (MgO) .
- (CS/OC, CR/OC ..) — , . — . :
( , - ) // / . OC/CS/CR , . , (= ).
.. , ) - ~200 ) )- ( ). -> , , , , , .
5. / any topical ophthalmic anesthetic, such as lidocaine (as in keratitis), can be instilled into the eyes. If, after the initial decontamination procedure, the symptoms of the lesion persist for a long time, it is necessary to deliver the victim to the nearest medical facility.
about an antidote against tear gas from Soviet childhoodRU Wikipedia (2005 , ) CS ( ) — .. . , — , , - . , … , .
UPD. vvz732 c : 0,894 , 0,621 , 0,598 , 0,04 .
, . . - — "… , , , , " NIOSH . . — . , — . . , -, . .
And remember that despite all the measures mentioned above, it is better to prevent the disease than to cure it. Be careful and prudent!
Many thanks to all my Patreon subscribers for their support and constructive criticism. To all active members of the LAB-66 community - my deep gratitude for their activity and unquenchable technical interest.
Remember, dear reader, that if you suddenly have any questions after the article, you can always discuss them in our telegram channel or in the LAN on Patreon .
1. 5536 0800 1174 5555
2. (QIWI) 79176005394
3. 410018843026512
4. 650377296748
5. BTC: 3QRyF2UwcKECVtk1Ep8scndmCBoRATvZkx, ETH: 0x3Aa313FA17444db70536A0ec5493F3aaA49C9CBf
6. steanlab!

.H., .. /M.: , 1990. — 271 .
McMahon, S. B., & Wood, J. N. (2006). Increasingly Irritable and Close to Tears: TRPA1 in Inflammatory Pain. Cell, 124(6), 1123–1125. doi:10.1016/j.cell.2006.03.006
Govindarajan, Sathyanarayana (1991). «Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences». Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
Howard L. Constant, Geoffrey A. Cordell and Dennis P. West (1996). «Nonivamide, a Constituent of Capsicum oleoresin». J. Nat. Prod. 59 (4): 425–426. doi:10.1021/np9600816
Rohm, Barbara; Riedel, Annett; Ley, Jakob P; Widder, Sabine; Krammer, Gerhard E; Somoza, Veronika (2015). «Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme a synthetase activity in Caco-2 cells». Food & Function. 6: 172. doi:10.1039/C4FO00435C
Rohini J. Haar, Vincent Iacopino, Nikhil Ranadive, Sheri D. Weiser & Madhavi Dandu, Health impacts of chemical irritants used for crowd control, BMC Public Health (2017), 17: 831.
Bennett DJ, Kirby GW (1968). «Constitution and biosynthesis of capsaicin». J. Chem. Soc. C: 442. doi:10.1039/j39680000442
Thompson, Robert Q (2007). «Homocapsaicin: Nomenclature, indexing and identification». Flavour and Fragrance Journal. 22 (4): 243. doi:10.1002/ffj.1814.
Olajos EJ, Salem H (2001). «Riot Control Agents: Pharmacology, Toxicology, Biochemistry and Chemistry». J Appl Toxicol. 21 (5): 355–391. doi:10.1002/jat.767. PMID 11746179
Jordt, Sven-Eric; Julius, David (February 2002). «Molecular Basis for Species-Specific Sensitivity to 'Hot' Chili Peppers». Cell. 108 (3): 421–430. doi:10.1016/S0092-8674(02)00637-2. PMID 11853675.
Rice Leonard M., Grogan Charles H., Armbrecht Bernard H., Reid E. Emmet. Pungents. Fatty Acid Amides1 // Journal of the American Chemical Society. — 1954. — (. 76, № 14). — . 3730—3731. — ISSN 0002-7863. — doi:10.1021/ja01643a043
Ditter, J. M., Heal, C. S. (2004). Application and use of riot control agents. (In) E. J. Olajos, W. Stopford (Eds.), Riot control agents issues in toxicology, safety, and health (pp. 17–24). Boca Raton: CRC Press LLC.
Kluchinsky, T. A., Sheely, M. V., Savage, P. B., Smith, P. A. (2002). Formation of 2-chlorobenzylidenemalononitrile (CS riot control agent) thermal degradation products at elevated temperatures. Journal of Chromatography A, 952(1–2), 205–213. doi.org/10.1016/S0021-9673(02)00096-1.
Olajos, E. J., Lakoski, J. M. (2004). Pharmacology/toxicology of CS, CR, CN, formulations, degradation products, carriers/solvents, and propellants. (In) E. J. Olajos, W. Stopford (Eds.), Riot control agents issues in toxicology, safety, and health (pp. 79–122). Boca Raton: CRC Press LLC.
Olajos, E. J., Salem, H. (2001). Riot control agents: Pharmacology, toxicology, biochemistry and chemistry. Journal of Applied Toxicology, 21, 355–391. doi.
org/10.1002/jat.767.
Schep, L. J., Slaughter, R. J., McBride, D. I. (2015). Riot control agents: The tear gases CN, CS and OC – a medical review. Journal of the Royal Army Medical Corps, 161(2),
94–99. doi.org/10.1136/jramc-2013-000165.
Smith, J., Greaves, I. (2002). The use of chemical incapacitant sprays: a review. The Journal of Trauma Injury, Infection and Critical Care, 52(3), 595–600.
Spicer, O., Almirall, J. R. (2005). Extraction of capsaicins in aerosol defense sprays from fabrics. Talanta, 67, 377–382. doi.org/10.1016/j.talanta.2005.05.031.
McMahon, S. B., & Wood, J. N. (2006). Increasingly Irritable and Close to Tears: TRPA1 in Inflammatory Pain. Cell, 124(6), 1123–1125. doi:10.1016/j.cell.2006.03.006
Govindarajan, Sathyanarayana (1991). «Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences». Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
Howard L. Constant, Geoffrey A. Cordell and Dennis P. West (1996). «Nonivamide, a Constituent of Capsicum oleoresin». J. Nat. Prod. 59 (4): 425–426. doi:10.1021/np9600816
Rohm, Barbara; Riedel, Annett; Ley, Jakob P; Widder, Sabine; Krammer, Gerhard E; Somoza, Veronika (2015). «Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme a synthetase activity in Caco-2 cells». Food & Function. 6: 172. doi:10.1039/C4FO00435C
Rohini J. Haar, Vincent Iacopino, Nikhil Ranadive, Sheri D. Weiser & Madhavi Dandu, Health impacts of chemical irritants used for crowd control, BMC Public Health (2017), 17: 831.
Bennett DJ, Kirby GW (1968). «Constitution and biosynthesis of capsaicin». J. Chem. Soc. C: 442. doi:10.1039/j39680000442
Thompson, Robert Q (2007). «Homocapsaicin: Nomenclature, indexing and identification». Flavour and Fragrance Journal. 22 (4): 243. doi:10.1002/ffj.1814.
Olajos EJ, Salem H (2001). «Riot Control Agents: Pharmacology, Toxicology, Biochemistry and Chemistry». J Appl Toxicol. 21 (5): 355–391. doi:10.1002/jat.767. PMID 11746179
Jordt, Sven-Eric; Julius, David (February 2002). «Molecular Basis for Species-Specific Sensitivity to 'Hot' Chili Peppers». Cell. 108 (3): 421–430. doi:10.1016/S0092-8674(02)00637-2. PMID 11853675.
Rice Leonard M., Grogan Charles H., Armbrecht Bernard H., Reid E. Emmet. Pungents. Fatty Acid Amides1 // Journal of the American Chemical Society. — 1954. — (. 76, № 14). — . 3730—3731. — ISSN 0002-7863. — doi:10.1021/ja01643a043
Ditter, J. M., Heal, C. S. (2004). Application and use of riot control agents. (In) E. J. Olajos, W. Stopford (Eds.), Riot control agents issues in toxicology, safety, and health (pp. 17–24). Boca Raton: CRC Press LLC.
Kluchinsky, T. A., Sheely, M. V., Savage, P. B., Smith, P. A. (2002). Formation of 2-chlorobenzylidenemalononitrile (CS riot control agent) thermal degradation products at elevated temperatures. Journal of Chromatography A, 952(1–2), 205–213. doi.org/10.1016/S0021-9673(02)00096-1.
Olajos, E. J., Lakoski, J. M. (2004). Pharmacology/toxicology of CS, CR, CN, formulations, degradation products, carriers/solvents, and propellants. (In) E. J. Olajos, W. Stopford (Eds.), Riot control agents issues in toxicology, safety, and health (pp. 79–122). Boca Raton: CRC Press LLC.
Olajos, E. J., Salem, H. (2001). Riot control agents: Pharmacology, toxicology, biochemistry and chemistry. Journal of Applied Toxicology, 21, 355–391. doi.
org/10.1002/jat.767.
Schep, L. J., Slaughter, R. J., McBride, D. I. (2015). Riot control agents: The tear gases CN, CS and OC – a medical review. Journal of the Royal Army Medical Corps, 161(2),
94–99. doi.org/10.1136/jramc-2013-000165.
Smith, J., Greaves, I. (2002). The use of chemical incapacitant sprays: a review. The Journal of Trauma Injury, Infection and Critical Care, 52(3), 595–600.
Spicer, O., Almirall, J. R. (2005). Extraction of capsaicins in aerosol defense sprays from fabrics. Talanta, 67, 377–382. doi.org/10.1016/j.talanta.2005.05.031.
